Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan F. Asturiano | + 3053 word(s) | 3053 | 2021-07-28 12:21:40 | | | |
| 2 | Amina Yu | Meta information modification | 3053 | 2021-07-29 05:56:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Asturiano, J.F. Reproductive Anatomy of Chondrichthyans. Encyclopedia. Available online: https://encyclopedia.pub/entry/12538 (accessed on 09 February 2026).
Asturiano JF. Reproductive Anatomy of Chondrichthyans. Encyclopedia. Available at: https://encyclopedia.pub/entry/12538. Accessed February 09, 2026.
Asturiano, Juan F.. "Reproductive Anatomy of Chondrichthyans" Encyclopedia, https://encyclopedia.pub/entry/12538 (accessed February 09, 2026).
Asturiano, J.F. (2021, July 28). Reproductive Anatomy of Chondrichthyans. In Encyclopedia. https://encyclopedia.pub/entry/12538
Asturiano, Juan F.. "Reproductive Anatomy of Chondrichthyans." Encyclopedia. Web. 28 July, 2021.
Copy Citation
Sperm extraction and artificial insemination may serve ex situ conservation initiatives for threatened sharks and related species. A comparison of the reproductive anatomy of eight chondrichthyans is presented in this study, emphasizing the important differences when performing these reproductive techniques. Additionally, we show how to obtain sperm samples from both living and dead specimens using cannulation, abdominal massage, or oviducal gland extraction. These tools can improve the success of breeding programs developed in aquaria and research facilities.
fish reproduction
aquarium
ex situ conservation
reproductive assisted techniques
artificial insemination
1. Introduction
The Chondrichthyes are a group of vertebrates that appeared more than 400 million years ago. Nowadays, this group is an ecologically diverse group with great importance in the regulation of the ecosystems where these animals inhabit [1][2]. The class Chondrichthyes comprises 1472 species classically divided into the holocephalans (subclass Holocephali), which are commonly named chimaeras, and the elasmobranchs (subclass Elasmobranchii), commonly named sharks and rays [3]. The chimaeras are the smallest of these three divisions in terms of the number of extant species, and currently there are 57 species described [3]. All chimaeras are marine animals, and very few species inhabit shallow waters, most species relegated to the deep waters (>200 m) despite their global distribution in the past [4]. Chimaeras have significantly different features compared to the elasmobranchs, such as the fusion of the lower jaw to the cranium (hence their name Holocephalans, “complete heads”) and non-replaceable tooth plates as teeth [4]. In general, holocephalans are a less studied group than their relatives, the rays and sharks. This last group, the sharks, is perhaps the most recognizable group among Chondrichthyans despite not being as numerous in terms of the number of species as the group formed by the rays and skates [5][6].
Regarding their conservation status, Chondrichthyes possess life histories that make them sensitive to elevated anthropic pressure, threatening their populations [7][8]. In fact, chondrichthyan extinction risk is higher than for most other vertebrates, and only one-third of the species assessed are considered safe according to IUCN red list criteria [9]. The situation is particularly sensitive in places such as the Mediterranean Sea, a key hotspot of extinction risk, where half the species of rays and sharks face an elevated risk of extinction [10]. Among the drivers for the global decline of its populations, overfishing (intentional or incidental) and habitat destruction are the main causes [9][10][11].
As mentioned above, to understand the current global situation of chondrichthyan populations, their reproductive strategies and life histories should be noted. Sharks and their relatives show larger body sizes, slower sexual maturity, longer gestation periods, higher maternal investment, and fewer offspring than other fishes [11][12]. Chondrichthyan species reproduction modes are diverse and can be divided according to the nutrition of the embryos. Lecithotrophic modes include oviparity (such as the catsharks of the family Scyliorhinidae, or the entire subclass Holocephali) and yolk sac viviparity (such as Hexanchiformes), where the only nourishment comes from their yolk sack. Matrotrophic modes include an additional nourishment source at some point of the embryo development, in the form of lipid histotrophy, unfertilized eggs (or fertilized eggs in the extreme case of the sand tiger shark Carcharias taurus), or the formation of a placenta (such as some Carcharhiniformes) [13][14][15].
In aquaculture industries, the reproductive factors and complex life histories mentioned above have discouraged captive breeding programs [13], but not in aquaria facilities, either public or private. The reproduction in captivity of elasmobranchs and chimaeras has been reported for some species [16][17][18], but these events have traditionally relied more on natural mating rather than on the use of reproductive techniques [19]. A potentially useful technique in breeding programs is the artificial insemination of females, but to ensure its success, a reliable supply of sperm is required, especially in the case of endangered species [19][20][21][22][23][24][25]. Although the obtention of sperm has been previously achieved for several shark and chimaera species [19][24][25][26][27][28][29], the procedures of extraction may vary between the different groups. In live animals, the most common procedures for sperm obtention have been cannulation and abdominal massage [19][22][29], but these techniques should consider the morphology and location of the reproductive structures, such as the seminal vesicles and urogenital papillae, to be truly effective.
Due to their position as one of the oldest groups of vertebrates [30], Chondrichthyes have been previously used as animal models for physiological and morphological studies [31][32][33][34]. Moreover, certain aspects of their reproductive morphology, such as the form and function of their intromittent organs [35][36][37][38] or gonads [39][40][41][42][43][44][45][46], have received attention from researchers and are well studied. However, some details about the morphology of certain reproductive structures, which are important during the use of reproductive techniques, have not been previously considered for sharks and chimaeras. Thus, the aim of this study is to offer an anatomical guide intended to be useful during sperm obtention procedures, as well as propose preliminary indications to be considered during artificial insemination. This practical guide complements previous work focused on the anatomy of batoids (rays, skates, and close species) and the use of reproductive techniques on them [47]. The tools presented in both studies are intended to delve into the development of reproductive techniques, such as sperm cryopreservation [29] or artificial insemination [25], some of the important steps toward successful and sustainable breeding programs.
2. Female General Anatomy: Sharks
It should be noticed that there is a wide variety of anatomical differences between the species studied, but this study aims to highlight only those that may be important while using specific techniques, such as sperm extraction and artificial insemination. Thus, other anatomical differences have not been considered. However, even though the abdominal pores are not part of the reproductive system, its misidentification may cause errors during cannulation, so its description is offered.
Externally, shark females possess a cloaca located between the pelvic fins, where the urinary, reproductive, and digestive system converge (Figure 1). Next to the caudal margin of the cloaca, the abdominal (or celomic) pores are found. These small orifices closed by a sphincter connect the pleuroperitoneal cavity with the exterior and may allow the removal of fluid from the inner cavity [48]. In females, the urinary system ends in the urinary papilla (the term urogenital papilla should be limited to males). Inside the cloaca, the caudal end of the uteri can be independent or converge and fuse in a common cervix (or urogenital sinus). The cranial orifice inside the cloaca is the caudal section of the digestive system: the rectum and the anus.
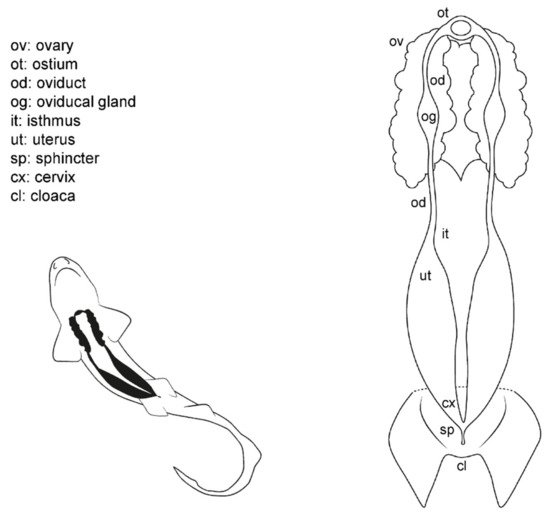
Figure 1. Female general anatomy: sharks. Morphological scheme of an ideal female shark, showing the main reproductive structures: ovary (ov), oviducts (od), ostium (ot), oviducal glands (og), isthmus (it), uterus (ut), sphincter (sp), cervix (cx), and cloaca (cl).
Internally, females possess a pair of reproductive tracts distributed along with the entire pleuroperitoneal space. The ovaries are embedded in the epigonal gland and located on the cranial part of the pleuroperitoneal cavity. Depending on the species, the ovaries can be paired, fused in a single ovary or vestigial, with only one ovary developed [13][15][38]. The oogenesis is produced in the ovaries, and the mature oocytes are released into the celoma and transported to the ostium by the action of cilia. The ostium is a funnel-like structure to allow the passage of the unfertilized oocytes into the anterior part of the oviduct and, then, to the oviducal gland (the terms shell gland and nidamental gland are commonly used, although in sharks, eggshells or embryo envelopes are not always produced). That gland is responsible for the storage of the sperm, the oocyte fertilization, and the formation of the egg case [49]. Thus, in oviparous species, the shell gland is proportionally bigger than in ovoviviparous or viviparous species. The gland also supposes the terminological division of the oviduct in the anterior (pre-oviducal gland) and posterior (post-oviducal gland) oviduct. In some species, a sphincter-like structure, the isthmus, can seal the oviducal gland from the uterus. Internally, females have one or two functional uteri depending on the species [13][15][38], where the embryos develop, or the eggs are kept until they are laid.
3. Female Anatomy: Chimaera monstrosa
Externally, C. monstrosa does not possess a common chamber (cloaca) where the digestive, urinary, and reproductive systems converge, but a single opening for the caudal portion of the digestive system, located between the pelvic fins (Figure 2) [46]. Two small pores closed by a sphincter sit along with this orifice, the abdominal (or celomic) pores that allowed an exchange of fluids between the exterior and the pleuroperitoneal cavity [48]. The entrances to the uteri are two independent orifices closed by a sphincter located at the base of the pectoral fins. In juvenile females, the orifices are narrow and hard to find, while in mature females, they are easier to locate.
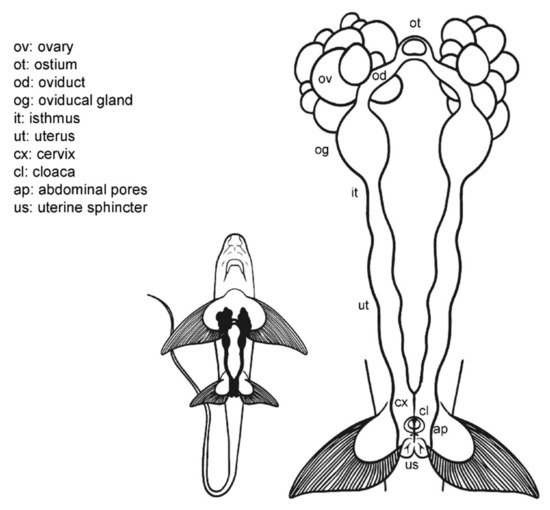
Figure 2. Female general anatomy: Chimaera monstrosa. Morphological scheme of C. monstrosa main female reproductive structures: ovary (ov), ostium (ot), oviduct (od), oviducal gland (og), isthmus (it), uterus (ut), cervix (cs), cloaca (cl), abdominal pores (ap), and uterine sphincter (us).
Internally, their reproductive system is composed of paired structures arranged longitudinally, in the same way as in the general model for sharks and rays. Following the longitudinal axis, two ovaries, a single ostium, two oviducts (divided into anterior and posterior) with an oviducal gland each, two isthmus, two functional uteri ended by the cervix, and a sphincter in the posterior section can be found. As in the rest of oviparous chondrichthyans [50], the oviducal gland of C. monstrosa is well developed, as at present, all chimaera species lay hard egg cases, despite their other past reproductive strategies [51].
4. Male General Anatomy: Sharks
Externally, male sharks have paired prolongations of the pelvic fins called claspers (or myxopterygium) used as intromittent organs for internal fertilization [13][34][35][36][37] (Figure 3). Claspers are located at the inner margin of the pelvic fins, forming a tube-like structure with a ventral groove known as hypopyle. The sperm flows through this groove from the male urogenital papilla to the female cloaca, and then into the uterine sphincter. To impulse the sperm, most sharks possess paired muscular structures at the base of the claspers, under the ventral skin, called siphon sacs [37][52]. The siphon sacs secrete serotonin (5-hydroxytryptamine), capable of producing muscle contractions in the uterus of females, which could favor the intrauterine transport of sperm [52]. Because multiple mating in sharks is well known, it has also been proposed that siphon sacs may play a role in sperm competition by washing rival sperm from the cloaca of females [53][54].
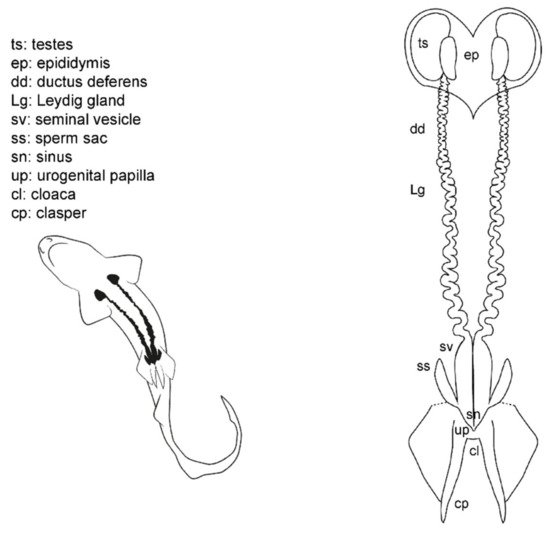
Figure 3. Male general anatomy: sharks. Morphological scheme of an ideal shark male, showing the main reproductive structures: testes (ts), epididymis (ep), ductus deferens (dd), Leydig gland (Lg), seminal vesicle (sv), sperm sac (ss), sinus (sn), urogenital papilla (up), cloaca (cl), and clasper (cp).
The cloaca is located between the pelvic fins. Typically, it is covered by the inner margins of the pelvic fins (the metapterygia), or in some species by two cloacal lips. Inside the cloaca are located the anus (caudal portion of the digestive system) and the urogenital papilla. Depending on the species, on the tip of the urogenital papilla, there are one or two pores through which the sperm and urine flow. Lastly, two abdominal pores connecting the pleuroperitoneal cavity with the exterior are located inside or near the cloaca, closed by a sphincter [48].
Internally, the male reproductive system is located along the pleuroperitoneal cavity. The paired testes, embedded in the epigonal organ, are located at the cranial end of the reproductive tract. The testes are connected via efferent ductules (or ductuli efferentes) with two genital tracts composed of the cranial convoluted part called epididymis, the ductus deferens (also called the Wolffian duct or vas deferens) [13], and the caudal part called seminal vesicle (or ampulla) where the sperm is stored. The Leydig gland is adjacent to the ductus deferens and empties its content on it and on the epididymis. The gland is formed by the cranial part of the mesonephros and produces the seminal fluid and the matrix where the spermatozeugmata, or the spermatophores, are formed [14][37][38][55]. In some species, a pouch-like structure can be found on the ventral surface of the seminal vesicle, the sperm sac, where mature sperm is stored until mating [41].
5. Male Anatomy: Chimaera monstrosa
The internal male reproductive system in C. monstrosa (Figure 4) is similar to that of sharks in many aspects [56]. Two paired testes located in the cranial region of the pleuroperitoneal cavity are connected with two genital tracts (which includes the epididymis, the ductus deferens with the Leydig gland and the seminal vesicle) by efferent ductules (or ductuli efferentes). The two seminal vesicles (or green glands [57]) are divided by an isthmus into a cranial section, first whitish and opaque and then greenish and translucent, and a grayish posterior section. The greenish coloration of the middle part of the seminal vesicle is a trait present in Chimaeriformes [46][57][58]. These caudal sections converge in a common sinus before forming the urogenital papilla in the exterior of the body.
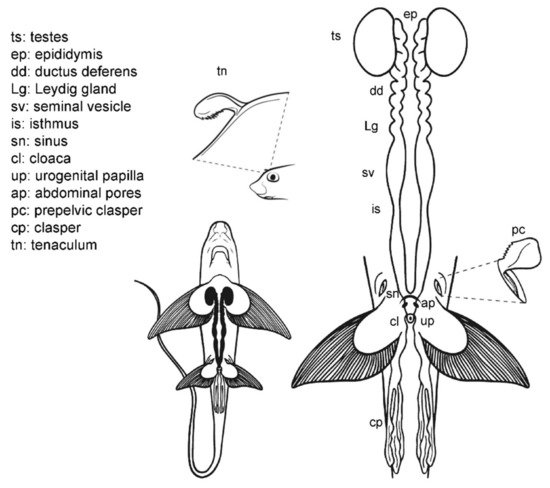
Figure 4. Male general anatomy: Chimaera monstrosa. Morphological scheme of the main reproductive male structures of C. monstrosa: testes (ts), epididymis (ep), ductus deferens (dd), Leydig gland (Lg), seminal vesicle (sv), isthmus (is), sinus (sn), cloaca (cl), urogenital papilla (up), abdominal pores (ap) and claspers [cephalic or tenaculum (tn), prepelvic (pc) and pelvic (cl)].
The external reproductive traits are multiple in C. monstrosa. First, the claspers are located at the base of the pelvic fins and receive the sperm from the external urogenital papilla. The claspers are three-lobed, with dermal denticles along its terminal region. Moreover, in C. monstrosa males, there are two slits located in the cranial part of the pelvic fins. In juvenile males, the slits are small and almost closed, but in adult males, the slits house prepelvic claspers, two serrated blade-like structures that emerge to grab the female during mating [18][46][59]. Another reproductive external trait is the cranial tenaculum. The tenaculum is a single mallet-like structure in the forehead of male chimaeras, with dermal denticles in its extreme. As in the case of prepelvic claspers, the tenaculum is used during reproduction to grab the females [18].
6. Male Comparative Anatomy
The overall morphology of the structures which can be relevant during sperm extraction is well preserved in all the species studied (Figure 5). However, there are some significant differences. Members of the family Scyliorhinidae studied (S. canicula, S. stellaris, G. melastomus) are quite similar (Figure 5A). The three species have two independent seminal vesicles converging in a single sinus and urogenital papilla, but both Scyliorhinus species possess abdominal pores near the inner margin of the pelvic fins, while the abdominal pores in G. melastomus are slightly more centered near the cloaca. The siphon sacs in the three species are also visible under the skin cranial to the pelvic fins. In P. glauca the siphon sacs are also easily visible and are clearly associated with each clasper, but there are several morphological differences with the previous group. In this species (Figure 5B) the abdominal pore takes the form of a papilla (abdominal papilla) located outside the cloaca. Inside, the two caudal parts of the deferent ducts converge in a wide common urogenital sinus along the ureters of the urinary system. Both the urogenital sinus and the deferens ducts are capable of storing a large amount of sperm. The urogenital sinus opens to the exterior through the urogenital papilla located in the middle of the cloaca. Even with these differences, the species are close relatives and belong to the same order Carcharhiniformes.
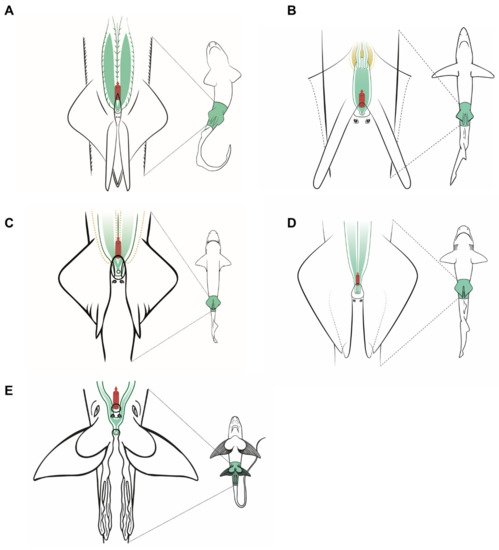
Figure 5. Specific male anatomy. Species-specific morphologies in sharks and C. monstrosa with relevance in sperm extraction procedures. The reproductive, excretory, and digestive systems are marked with different colors: the seminal vesicle and ductus deferens in green, the excretory system in yellow, and the access to the digestive system in red. The dotted lines in Centrophorus uyato represent the position of the hepatic lobes. The grey circles are the abdominal pores that connect the pleuroperitoneal cavity with the exterior. (A) Model observed in catsharks, family Scyliorhinidae. (B) Model for blue shark Prionace glauca. (C) Model observed in little gulper shark Centrophorus uyato. (D) Bluntnose sixgill shark Hexanchus griseus. (E) Rabbitfish Chimaera monstrosa.
In the case of the little gulper shark (Centrophorus uyato), a deep-water shark that belongs to the order Squaliformes, the internal reproductive structures (Figure 5C) closely resemble those of the previous models observed: two paired testes, reproductive ducts, and independent seminal vesicles converging in a urogenital sinus. The external reproductive traits, however, showed several differences. The cloaca is partially covered by two cloacal lips, and only the tip of the urogenital papilla is visible if the lips are not separated. Claspers are proportionally shorter and thinner, with internal spines that unfold inside the female tract during mating. Moreover, siphon sacs are not visible in the ventral region, as in the previous species, but there are two folds under the ventral surface of the pelvic fins (also called siphons) with the same function. The absence of ventral siphon sacs also occurs in the bluntnose sixgill shark (Hexanchus griseus), another deep-water species belonging to the order Hexanchiformes (Figure 5D). In this species, a sac-like structure along the clasper (called clasper sac) can be found. This structure, a unique feature of Hexanchiformes, can inflate and function like the siphon sac of other elasmobranchs. Moreover, in this species the claspers lie in the inner rear margin of the pelvic fins which form a scroll, absent in females [60]. As in C. uyato, the abdominal pores in H. griseus are located at the base of the cloaca, but in this species as two abdominal papillae. Unlike the rest of the species studied, the ductus deferens and seminal vesicles do not converge in a common urogenital sinus. Instead, the two reproductive tracts are independent even in the urogenital papilla, where two different pores are located, one for each tract. This difference has also been described in the broadnose sevengill shark (Notorynchus cepedianus) [61], another member of the Hexanchiformes, suggesting that it could be a common trait for this order.
C. monstrosa is the only holocephalan studied, but with some exceptions, the overall internal morphology of the reproductive system is shared with the rest of the species observed (Figure 5E). The epigonal organ, present in elasmobranchs, is absent or cannot be easily identified in C. monstrosa and probably in the rest of holocephalans [46][57][55][62]. The division of the seminal vesicle into two sections separated by an isthmus does not occur in elasmobranchs but appears in other holocephalans such as the spotted ratfish (Hydrolagus colliei [62]) and H. melanophasma [57], though neither in C. callorhynchus [63] nor Chimaera phantasma [58]. The greatest difference between C. monstrosa and the rest of species studied appears when observing their external reproductive traits such as the clasper morphology, the absence of cloaca, and the presence of prepelvic claspers and tenaculum.
References
- Compagno, L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 1990, 28, 33–75.
- Stevens, J. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494.
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. CAS-Eschmeyer’s Catalog of Fishes. Available online: (accessed on 7 April 2021).
- Didier, D.A. Phylogeny and classification of extant Holocephali. In Biology of Sharks and Their Relatives; Heithaus, M.R., Musick, J.A., Carrier, J.C., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 115–138.
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish. Biol. 2016, 88, 837–1037.
- Fowler, S.L.; Cavanagh, R.D. Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes: Status Survey; IUCN: Gland, Switzerland, 2005; Volume 63, ISBN 2831707005.
- García, V.B.; Lucifora, L.O.; Myers, R.A. The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc. B Biol. Sci. 2008, 275, 83–89.
- Dulvy, N.K.; Forrest, R.E. Life histories, population dynamics and extinction risks in chondrichthyans. In Biology of Sharks and Their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, pp. 639–679.
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. Elife 2014, 3, e00590.
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea (Brochure); IUCN: Malaga, Spain, 2016.
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571.
- Cortés, E. Life History Patterns and Correlations in Sharks. Rev. Fish. Sci. 2000, 8, 299–344.
- Walker, T.I. Reproduction of Chondrichthyans. In Reproduction in Aquatic Animals; Yoshida, M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 193–223.
- Musick, J.A.; Ellis, J.K.; Hamlett, W. Reproductive evolution of chondrichthyans. In Reproductive Biology and Phylogeny of Chondrichthyes, Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 45–71.
- Conrath, C.L.; Musick, J.A. Reproductive biology of elasmobranchs. In Biology of Sharks and Their Relatives, 2nd ed.; Carrier, J.D., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2010; Volume 2, pp. 291–311.
- Janse, M.; Zimmerman, B.; Geerlings, L.; Brown, C.; Nagelkerke, L.A.J. Sustainable species management of the elasmobranch populations within European aquariums: A conservation challenge. J. Zoo Aquar. Res. 2017, 5, 172–181.
- Henningsen, A.D.; Smale, M.J.; Gordon, I.; Garner, R.; Marin-Osorno, R.; Kinnunen, N. Captive breeding and sexual conflict in elasmobranchs. In The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2004; pp. 237–248.
- Barnett, L.A.K.; Earley, R.L.; Ebert, D.A.; Cailliet, G.M. Maturity, fecundity, and reproductive cycle of the spotted ratfish. Mar. Biol. 2009, 301–316.
- Daly, J.; Jones, R. The use of reproductive technologies in breeding programs for elasmobranchs in aquaria. In The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and Their Relatives; Smith, M., Warmolts, D., Thoney, D., Hueter, R., Murray, M., Ezcurra, J., Eds.; Special Publication of the Ohio Biological Survey: Columbus, OH, USA, 2017; pp. 363–374.
- Luer, C.A.; Walsh, C.J.; Bodine, A.B.; Wyffels, J.T. Normal embryonic development in the clearnose skate, Raja eglanteria, with experimental observations on artificial insemination. In Biology of Skates; Ebert, D.A., Sulikowski, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 133–149.
- Daochai, C.; Keschumras, N.; Chansue, N.; Haetrakul, T. Preliminary of intra-vagina artificial insemination using fresh semen in Ocellate river stingray (Potamotrygon motoro). Thai J. Vet. Med. 2020, 50, 383–385.
- Penfold, L.M.; Wyffels, J.T. Reproductive science in sharks and rays. Adv. Exp. Med. Biol. 2019, 1200, 465–488.
- Masuda, M.; Izawa, Y.; Kametuta, S.; Ikuta, H.; Isogai, T. Artificial insemination of the cloudy catshark. J. Jpn. Assoc. Zool. Gard. Aquar. 2003, 44, 39–43.
- Masuda, M.; Izawa, Y.; Kametsuta, S.; Ikuta, H.; Isogai, T. Artificial insemination of the white-spotted bamboo shark, Chiloscyllium plagiosum. J. Jpn. Assoc. Zool. Gard. Aquar. 2005, 46, 91–96.
- Wyffels, J.T.; Adams, L.M.; Bulman, F.; Fustukjian, A.; Hyatt, M.W.; Feldheim, K.A.; Penfold, L.M. Artificial insemination and parthenogenesis in the whitespotted bamboo shark Chiloscyllium plagiosum. Sci. Rep. 2021, 11, 9966.
- Minamikawa, S.; Morisawa, M. Acquisition, initiation and maintenance of sperm motility in the shark, Triakis scyllia. Comp. Biochem. Physiol. A Physiol. 1996, 113, 387–392.
- Pratt, H.L.; Tanaka, S.H.O. Sperm Storage in Male Elasmobranchs: A Description and Survey. J. Morphol. 1994, 308, 297–308.
- Wyffels, J.T.; George, R.; Adams, L.; Adams, C.; Clauss, T.; Newton, A.; Hyatt, M.W.; Yach, C.; Penfold, L.M. Testosterone and semen seasonality for the sand tiger shark Carcharias taurus. Biol. Reprod. 2020, 102, 876–887.
- García-Salinas, P.; Gallego, V.; Asturiano, J.F. Development of Sperm Cryopreservation Protocols for Sharks and Rays: New Tools for Elasmobranch Conservation. Front. Mar. Sci. 2021, 8, 689089.
- Wourms, J.P. Reproduction and development in chondrichthyan fishes. Am. Zool. 1977, 17, 379–410.
- Luer, C.A. Elasmobranchs (sharks, skates, and rays) as animal models for biomedical research. In Nonmammalian Animal Models for Biomedical Research; CRC Press: Boca Raton, FL, USA, 1989.
- Coolen, M.; Menuet, A.; Chassoux, D.; Compagnucci, C.; Henry, S.; Lévèque, L.; Da Silva, C.; Gavory, F.; Samain, S.; Wincker, P. The dogfish Scyliorhinus canicula: A reference in jawed vertebrates. Cold Spring Harb. Protoc. 2008, 2008.
- Lauriano, E.R.; Pergolizzi, S.; Gangemi, J.; Kuciel, M.; Capillo, G.; Aragona, M.; Faggio, C. Immunohistochemical colocalization of G protein alpha subunits and 5-HT in the rectal gland of the cartilaginous fish Scyliorhinus canicula. Microsc. Res. Tech. 2017, 80, 1018–1027.
- Luer, C.A.; Walsh, C.J. Potential human health applications from marine biomedical research with elasmobranch fishes. Fishes 2018, 3, 47.
- Leigh-Sharpe, W.H. The comparative morphology of the secondary sexual characters of Holocephali and elasmobranch fishes. The claspers, clasper siphons, and clasper glands. J. Morphol. 1922, 36, 199–220.
- Jungersen, H.F.E. On the Appendices Genitales in the Greenland Shark, Somniosus Microcephalus (BL. Schn.), and Other Selachians. In Danish Ingolf-Expedition, 1895-1896; Luno, B., Dreyer, F., Eds.; Bianco Luno: Copenhagen, Denmark, 1899; Volume 2.
- Gilbert, P.W.; Gordon, W.H. The clasper-siphon sac mechanism in Squalus acanthias and Mustelus canis. Comp. Biochem. Physiol. Part A Physiol. 1972, 42, 97–119.
- Jones, C.J.P.; Walker, T.I.; Bell, J.D.; Reardon, M.B.; Ambrosio, C.E.; Almeida, A.; Hamlett, W.C. Male genital ducts and copulatory appendages in chondrichthyans. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 361–393.
- Dodd, J.M. 2 Reproduction in Cartilaginous Fishes (Chondrichthyes). In Reproduction; Hoar, W.S., Randall, D.J., Donaldson, E.M.B.T.-F.P., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 9, pp. 31–95. ISBN 1546-5098.
- Del Mar Pedreros-Sierra, T.; Arrieta-Prieto, D.M.; Mejía-Falla, P.A. Reproductive system of females of the Magdalena river endemic stingray P otamotrygon magdalenae: Anatomical and functional aspects. J. Morphol. 2016, 277, 680–697.
- Chen, C.-T.; Teshima, K.; Mizue, K. Studies on Sharks―IV Testes and Spermatogeneses in Selachians. Bull. Fac. Fish. Nagasaki Univ. 1973, 35, 53–65.
- Parsons, G.R.; Grier, H.J. Seasonal changes in shark testicular structure and spermatogenesis. J. Exp. Zool. 1992, 261, 173–184.
- Davenport, I.R.; Weaver, A.L.; Wourms, J.P. A novel set of structures within the elasmobranch, ovarian follicle. J. Morphol. 2011, 272, 557–565.
- Lutton, B.V. The elasmobranch ovary. In Reproductive Biology and Phylogeny of Chondrichthyes; CRC Press: Boca Raton, FL, USA, 2011; pp. 247–276. ISBN 0429062907.
- Pratt, H.L., Jr. Elasmobranch gonad structure: A description and survey. Copeia 1988, 1988, 719–729.
- Dean, B. Chimæeroid Fishes and Their Development; Carnegie Institution of Washington: Washington, DC, USA, 1906.
- García-Salinas, P.; Gallego, V.; Asturiano, J.F. Reproductive Anatomy of Chondrichthyans: Notes on Specimen Handling and Sperm Extraction. I. Rays and Skates. Animals 2021, 11, 1888.
- De Iuliis, G.; Pulerà, D. The Dissection of Vertebrates, 2nd ed.; Academic Press: Oxford, UK, 2019; ISBN 0124105009.
- Hamlett, W.C.; Knight, D.P.; Koob, T.J.; Jezior, M.; Luong, T.; Rozycki, T.; Brunette, N.; Hysell, M.K. Survey of oviducal gland structure and function in elasmobranchs. J. Exp. Zool. 1998, 282, 399–420.
- Hamlett, W.C.; Koob, T.J. Female reproductive system. In Sharks, Skates and Rays: The Biology of Elasmobranch Fishes; Hamlett, W.C., Ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 398–443.
- Musick, J.A. Chondrichthyan reproduction. In Reproduction and Sexuality in Marine Fishes: Patterns and Processes; Cole, K.S., Ed.; University of California Press: Oakland, CA, USA, 2010; pp. 3–19. ISBN 9780520264335.
- Mann, T.; Prosser, L.C. Uterine response to 5-Hydroxytryptamine in clasper-siphon secretion of spiny dogfish, Squalus acanthias. Biological Bulletin: Woods Hole, MA, USA, 1963; Volume 125, pp. 384–385.
- Pratt, H.L.; Carrier, J.C. Elasmobranch Courtship and Mating Behavior. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids, and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 129–169.
- Whitney, N.M.; Pratt, H.L.; Carrier, J.C. Group courtship, mating behaviour and siphon sac function in the whitetip reef shark, Triaenodon obesus. Anim. Behav. 2004, 68, 1435–1442.
- Jamieson, B.G.M.; Hamlett, W.C. Chondrichthyan spermatozoa and phylogeny. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids, and Chimaeras; Hamlett, W.C., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 201–236.
- Bell, J.D. Reproduction and Ageing of Australian Holocephalans and White-Fin Swell Shark. Ph.D. Thesis, Deakin University, Melbourne, VIC, Australia, 2012.
- Márquez-Farías, J.F.; Lara-Mendoza, R.E. Notas sobre la morfología del aparato reproductor de la quimera, Hydrolagus melanophasma (Chondrichthyes, Holocephali), de la costa oeste de Baja California, México. Hidrobiológica 2014, 24, 151–158.
- Malagrino, G.; Takemura, A.; Mizue, K. Studies on Holocephali―II On the Reproduction of Chimaera phantasma Jordan et Snyder Caught in the Coastal Waters of Nagasaki. Bull. Fac. Fish. Nagasaki Univ. 1981, 51, 1–7.
- Kessel, S.T.; Hussey, N.E. Tonic immobility as an anaesthetic for elasmobranchs during surgical implantation procedures. Can. J. Fish. Aquat. Sci. 2015, 72, 1287–1291.
- Ebert, D.A. Some observations on the reproductive biology of the sixgill shark Hexanchus griseus (Bonnaterre, 1788) from South African waters. Afr. J. Mar. Sci. 2002, 24, 359–363.
- Daly, J. Clinical Studies. Available online: (accessed on 12 April 2021).
- Stanley, H.P. Urogenital morphology in the chimaeroid fish Hydrolagus colliei (Lay and Bennett). J. Morphol. 1963, 112, 99–127.
- Di Giacomo, E.E.; Perier, M.R. Reproductive biology of the cockfish, Callorhynchus callorhynchus (Holocephali: Callorhynchidae), in Patagonian waters (Argentina). Fish. Bull. 1994, 92, 531–539.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.4K
Revisions:
2 times
(View History)
Update Date:
29 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




