
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Bialik | + 3925 word(s) | 3925 | 2021-05-25 08:02:45 | | | |
| 2 | Rita Xu | Meta information modification | 3925 | 2021-07-29 04:07:38 | | |
Video Upload Options
Thermosensitive gelling system is drug delivery system that becomes a gel at physiological temperature. The transition sol-gel temperature (Tsol-gel) is below body temperature (<37◦C), making it possible to prepare liquid preparations, which gel back at body temperature. Thermosensitive gelling system could be used to rectal administration. Rectal drug delivery is an effective alternative to oral and parenteral treatments. This route allows for both local and systemic drug therapy. Traditional rectal dosage formulations have historically been used for localised treatments, including laxatives, hemorrhoid therapy and antipyretics. However, this form of drug dosage often feels alien and uncomfortable to a patient, encouraging refusal. Thermosensitive liquid suppositories are easier to administer to the anus as they remain liquid at lower temperatures. In addition, this rectal gel minimize the feeling of a foreign body compared with solid suppositories, does not cause any harm on mucosal layers and act as mucoadhesive to the rectal tissues preventing leakage after administration.
1. Introduction
Oral administration is the preferred route for regular pharmacotherapy, as it is the easiest and most convenient. However, this is not feasible or even impossible in some cases (e.g., during nausea/vomiting or convulsions, in non-cooperative patients, prior to surgery). In such situations, the rectal route can be a viable option, and rectal administration is now well known for drug delivery. Traditionally solid suppositories are the most popular delivery systems applied for rectal drug administration and account for more than 98% of all rectal dosage forms. Traditional solid-type suppositories, however, often feel alien and uncomfortable to patients, encouraging refusal. To solve these problems, it would be desirable to develop a thermosensitive liquid suppository[1][2]. Such a formulation is administered to the anus simply, does not damage the mucosal layers, behaves as mucoadhesive to the rectal tissue without leakage and reduces the feeling of a foreign body. Thermosensitive liquid suppositories can be used for a variety of drugs, such as anticancer, analgesic, etc. Furthermore, thermosensitive systems enable superior control of drug release by varying the type and concentration of components. This solution may have a positive effect on pharmacotherapy effectiveness. The properties of this dosage form depend on the type of components used (thermosensitive and mucoadhesive polymers) and their concentration [3].
1.1. Oral Route and Its Limitations
Many drugs are intended for oral use in the pharmaceutical industry. Oral drug delivery accounts for more than 50% of the global demand for drug delivery. The oral route (per oral (p.o.) is the most favoured route for drug administration, because it offers the highest degree of patient compliance. The benefits of oral administration are as follows:
- convenient — can be self-administered, pain-free, easy to take;
- absorption — occurs along the entire length of the gastrointestinal tract (GIT);
- cheap — compared to most other drug formulations [1][2].
For an oral drug, the time to onset of a desired pharmacological effect depends on a number of successive steps: dissolution of the formulation, passage to the site of absorption, permeation through physiological membranes, entry into the portal vein circulation, distribution from plasma to the site of action, and interaction with the receptor [4][5].
Oral administration is very effective for drugs with high solubility and gastrointestinal permeability. However, effective oral drug delivery with poor solubility and/or permeability and/or metabolic stability is quite challenging. In general, these drugs must be administered at a high dose to achieve therapeutic concentration [2]. Furthermore, due to their susceptibility to extreme GIT conditions and the risk of chemical or enzymatic degradation, many of them have a low percentage of absorption via the oral route. The pH of the GIT varies with the location. For example, the stomach has an acidic pH, but the pH in the intestine is in the range of 6.8–7.4. Drugs, such as artemether, erythromycin, candesartan cilexetil, undergo chemical degradation at acidic pH. For this reason, their bioavailability is much lower [2]. In addition, the various enzymes (esterases, lipases) present in the GIT result in the degradation of several drugs, such as antihyperlipidemic agents (simvastatin, ezetimibe) and cephalosporin antibiotics (cefpodoxime proxetil). Oral drug bioavailability, such as antidiabetic agents (repaglinide) and antihypertensive and cardiovascular agents (β-blockers, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitors), is substantially low due to high levels of first-pass (hepatic) metabolism. Finally, drug efflux transporters such as P-glycoprotein are also responsible for the efflux of various drugs such as digoxin, paclitaxel, and doxorubicin from the site of absorption. It also reduces the bioavailability of the drugs [2][5][6][7][8].
In order to overcome these disadvantages, a number of formulation methods such as prodrugs (macromolecular conjugates), solid dispersions, self-microemulsifying drug delivery systems (SMEDDSs), microcapsules including liposomes, nanoparticles, P-glycoprotein inhibitor pre-treatment have been aimed to enhance drug physicochemical properties and to reach therapeutically relevant plasma drug concentrations [9][10][11][12][13]. However, the bioavailability of oral lipophilic medicines remains a major concern. Hence, there is a growing need to develop efficient DDSs to improve the bioavailability and therapeutic profile of a wide range of drugs. Among the many non-invasive routes available, the rectal route is a safe and promising alternative to drug delivery.
1.2. Rectal Route
Rectal dosage forms are the oldest pharmaceutical dosage forms seeing as their origin dates back to antiquity. Hippocrates mentions the different compositions of acorns that were rectal dosage types, and the Old Testament refers to “Magerarta”—a silver suppository. The first rectal dosage formulations consisted of solid supports (baked honey, soap, tallow, horn) impregnated with medicinal substances. These solid supports were replaced by cocoa butter at the end of the 18th century. The first mention of the addition of an active substance to the suppository mass was made by Henry and Guibourt in 1841 with the introduction of opium in cocoa butter [14].
In humans, the rectum is formed by the last 15 to 19 cm of the large intestine. The rectum has two or three curves within its lumen formed by submucosal folds [15]. The rectal wall is formed by an epithelium. Its luminal surface is covered by a membrane formed by 1 layer of cells, consisting of columnar epithelial, endocrine and goblet cells which secrete mucus. The surface area available for drug absorption in the rectum is approximately 200–400 cm2. The volume of fluid in the rectum is approximately 1–3 ml and is viscous. The rectal pH is constant and approximately 7.5–8.0; the temperature is usually 37 °C. The venous drainage of the rectum consists of three distinct veins. The upper haemorrhoid vein drains the upper rectum and empties into the haemorrhoidal vein, which flows into the liver. The middle and lower haemorrhoidal veins drain the rest of the rectum and return to the inferior vena cava (Figure 1). Absorption of water, sodium and chloride and secretion of potassium and bicarbonate occurs in the human colon, while active glucose and amino acid transport are lacking. Sodium and water absorption in the rectum is negligible [15][16][17] [18].
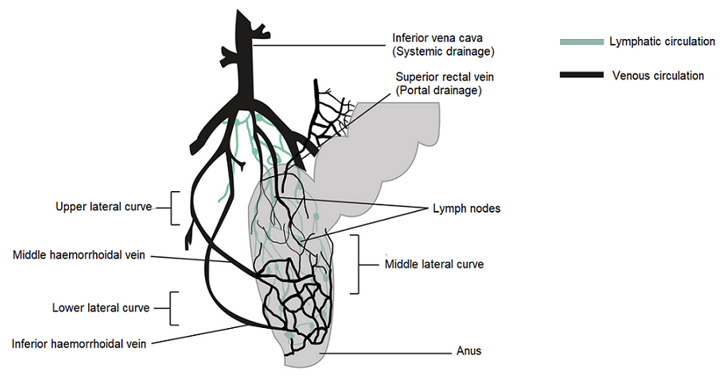
Figure 1. Venous and lymphatic drainage from the rectum.
The rectum has a relatively small absorption surface of 0.02–0.05 m2 and is characterized by a lack of villi. Drug absorption from rectal epithelium involves two transport routes: trans-cellular and para-cellular. The para-cellular route is the diffusion of drugs through space between epithelial cells, and the mechanism of uptake in the trans-cellular route depends on lipophilicity. Rectal drug absorption depends on several drug characteristics: small partition coefficient, large molecular size, charge and high capability of hydrogen bond formation are standard reasons for poor drug absorption [14]. The presence of faeces, which can also alter the absorption of the drug, is another hindrance. In the absence of faecal matter, the drug is more likely to come into contact with the absorbing rectal surface. Rectal content is usually alkaline; alkaline solutions are rapidly absorbed rather than acidic solutions. Other conditions, such as diarrhoea, colonic obstruction, and tissue dehydration, may influence the rate and degree of drug absorption from the rectal site. Aqueous and alcoholic solutions are absorbed rapidly, whereas suspensions and suppositories are absorbed slowly and constantly. The lower rectum is drained by the lower and middle haemorrhoidal veins and bypasses the liver, thereby avoiding, at least partially, the hepatic first-pass effect and enabling drugs to have systemic effects prior to metabolism in the liver. The rectal region is also massively drained by lymphatic circulation and may increase the systemic absorption of certain highly lipophilic drugs. Drug use through the rectal path is susceptible to both local and systemic drug delivery [14][19][20][21][22]. This route is used as an alternative to oral and invasive administration. Rectal drug delivery is essential if oral or intravenous treatment is not possible. Furthermore, it is ideally adapted for infants, children and geriatric patients [14][23][24].
The rectal route provides the following possible advantages for drug delivery over the oral route:
- rapid absorption of several drugs with low molecular weight;
- partial prevention of the first-pass metabolism, the potential for absorption into the lymphatic system;
- the retention of higher drug quantities;
- probability of efficient drug delivery and absorption [25][26];
- protection of enzymatically unstable drugs (insulin) owing to the lack of enzymes in the rectum [27][28];
- minimal first-pass drug metabolism given that the suppository is administered at an acceptable distance in the rectum [29];
- avoidance of exposure of gastric mucosa to irritant drugs such as non-steroidal anti-inflammatory drugs;
- increased effectiveness [30][31].
Conventional rectal dosage formulations are available in a number of countries. These forms have historically been used for localised treatments, including antipyretics, haemorrhoid and laxative therapy. Recent trends show an increase in the production of new rectal delivery systems to deliver drugs directly to the systemic circulation. In addition, higher bioavailability and controlled drugs release have become possible with the support of modern pharmaceutical products and innovative Rectal Drug Delivery Systems (RDDSs).
Such systems may be modified to act locally or systemically and may release the active agent immediately or over in an extended manner [16]. Traditional rectal dosage forms can be categorized into solid (suppository), liquid (enema, microenema, foam, suspension), semi-solid (gel, ointment) and medical devices (rectal tampon) [16]. Traditionally solid suppositories are the most common delivery systems used in rectal drug administration. They represent over 98% of all the rectal dosage forms. Suppositories containing a drug, solubilized or suspended in a suppository base, that upon melting or solubilization at physiological conditions, release the active substance for either local or systemic action [16][23][32]. Unfortunately, traditional solid suppositories suffer from disadvantages such as pain, an alien feeling and, thus, a patient’s refusal. A solid type suppository, which may reach the end of the colon, may also allow drugs carried to undergo the first-pass effect and many drugs are poorly or erratically absorbed across the rectal mucosa. It is also possible to metabolise drugs in microorganisms and rectal mucosa. In addition, certain suppositories are either “leaked” or expelled after insertion. In order to avoid these problems, the suppository should be inserted past the muscular sphincter, which is approximately half an inch in the rectum of infants and one inch in the rectum of children and adults [33][34]. In addition, the rectal absorption of most drugs is often erratic and unpredictable. The anus has a limited absorption surface area and may cause problems with dissolution due to the small fluid content of the rectum. In addition, in countries with a tropical climate, the proximity of its melting point to average room temperature is another drawback of conventional suppositories. Some of the problems in transport and storage could be observed. The optimal suppository should be easy to administer, without any pain during insertion, and should remain at the administered site to prevent the first-pass effect in the liver [19][23][35].
Traditional RDDSs have recently been improved by modifying the properties of the formulation, e.g., gelation temperature, gelation strength. Prolonged retention and controlled drugs release can help improve bioavailability and provide better pharmacokinetic profiles or local treatment effects. RDDS has been developed to provide greater control over the spread, retention and/or release of the drug through a range of formulation strategies. The types of novel RDDSs are as follows: hollow-type suppository, thermosensitive liquid suppository, mucoadhesive gel, micro and nanoparticles and vesicular drug delivery systems [16].
In particular, the problems of conventional solid suppositories related to the above can be solved by developing thermosensitive liquid suppositories. This material:
- forms a gel at a body temperature;
- has the required gel strength to prevent leaking out of the anus after administration;
- has sufficient bioadhesive force so as not to reach the end of the colon [16][22][36][37].
Furthermore, the simplicity of administration and the ability to reduce discomfort to the rectal mucosa has contributed to the development of these dosage forms [38][39].
1.3. Polymers and Their Properties Used in the Fabrication of Thermosensitive Liquid Suppositories
Liquid suppositories are thermosensitive rectal gels. They are also referred to as thermosensitive liquid suppositories due to the fact that the base material used in the formulation is a thermosensitive polymer that becomes a gel at physiological temperature (37 °C). The transition sol-gel temperature (Tsol-gel) is below body temperature (<37 °C), making it possible to prepare liquid preparations, which gel back at body temperature [16][40]. Thermosensitive polymers such as poloxamers or pluronics in a proper concentration form a gel at physiological temperature, thus preventing leakage and excessive spreading in the rectum. In addition, the combination of mucoadhesive and thermosensitive polymers allows for more sustained drug release relative to the thermosensitive polymer alone [31][39][41][42]. Once in the rectal cavity, the development of mucoadhesive properties helps to immobilize the hydrogel for a prolonged period of time and to prolong the drug release, thereby favouring the systemic absorption of drugs [43]. Viscosity, gelling time and temperature threshold are all main elements in the preparation of thermosensitive liquid suppository [16][44][45].
The key benefits of thermosensitive liquid suppositories over traditional or solid suppositories are as follows:
- they are easy to administer to the anus as they remain liquid at lower temperatures,
- act as mucoadhesive to the rectal tissues preventing leakage after administration,
- do not cause any harm on mucosal layers,
- minimize the feeling of a foreign body compared with solid suppositories [3][16][41].
The most popular base of thermosensitive liquid suppository are poloxamers (triblock copolymers of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) (PEO–PPO–PEO)) [46]. They comprise a central block of hydrophobic polypropylene oxide (PPO) surrounded on both sides by the blocks of hydrophilic polyethylene oxide (PEO) [46] (Figure 2a). Triblocks copolymers (Poloxamer® or Pluronic® series) are the most commonly encountered thermosensitive systems in the pharmaceutical field. Mucoadhesive and thermosensitive polymers have gained much attention recently to decrease patient discomfort and relieve the alien feeling due to solid suppository insertion, and afford an endurable method of administration. Poloxamer solutions exhibit the phenomenon of reverse thermal gelation and remaining as solutions at low temperature (4 °C) and gelling again upon raising temperature (25–35 °C) [11]. In general, the phase transition temperature of poloxamers depends on their concentration. Poloxamer aqueous solutions stay fluid below Tsol-gel, and the solution transforms to a semi-solid material above this temperature. Thermogelation is caused by hydrophobic interactions between the copolymer chains of poloxamer. When the temperature is raised, the poloxamer copolymer chains begin to aggregate into a micellar structure. The poloxamer in cold water acts as follows: the hydration layer surrounds the poloxamer molecule and the hydrophobic portion is separated due to hydrogen bonds. The hydrogen bonds break and the hydrophilic chains dissolve as the temperature increases. Poloxamer gelation is associated with a polymer dehydration process, which increases chain friction and entanglement while also producing hydrophobic association [3][38][47][48]. Poloxamers are also known for their compatibility with other compounds, high solubilization capacity of various active ingredients and good characteristics for active ingredient prolonged release [3][43][49][50].
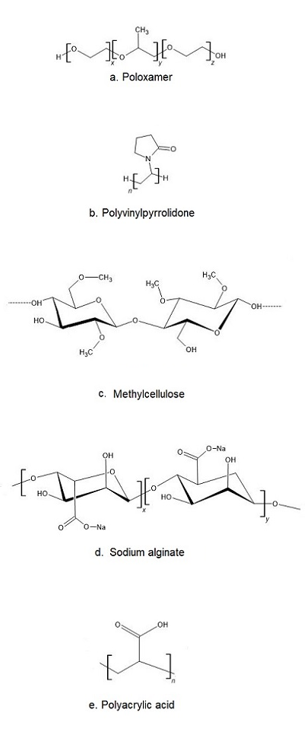
Figure 2. The chemical structure of exemplary polymers used in the production of thermosensitive liquid suppository: a) poloxamer, b) polivinylpyrrolidone, c) methylcellulose, d) sodium alginate, e) polyacrylic acid.
Poloxamer 407 (P407, Pluronic F 127) is widely used because it allows the formation of colourless, transparent and easily washable water gels, which are non-irritating to the skin and mucous membranes. Its transition temperature is below body temperature, allowing for the preparation of liquid preparations that gel at body temperature. At ambient temperature, an aqueous solution of P407 with a concentration higher than 20% forms non-chemically cross-linked hydrogels. P407 solution has a lower phase transition temperature (<25 °C) at such a concentration, so solutions are gels at room temperature and thus difficult to use for drug delivery [51][52]. In order to create a temperature-responsive gel with a suitable phase transition temperature, poloxamer 188 (P188) is incorporated into P407 solutions to modulate the phase transition temperature [52][53]. The poloxamer mixture solutions have a higher transition temperature than the P407.
Despite many benefits, poloxamer hydrogels do not possess or have weak bioadhesive properties. In addition, they suffer a major disadvantage, which is low mechanical strength and high water permeability, limiting their use as a thermosensitive matrix [38]. If mucoadhesive properties are required, poloxamer must be formulated with other bioadhesive polymers. Other typical mucoadhesive polymers used in the preparation of liquid suppositories are: polyvinylpyrrolidone (PVP (Figure 2b)), sodium alginate, acrylic polymers (Polycarbophyl® (PCP)), Carbopol®, and cellulose ether polymers such as carboxymethyl cellulose (CMC), hydroxypropyl methylcellulose (HPMC, Hypromellose), methylcellulose (MC, Metolose® (Figure 2c)), hydroxyethyl cellulose (HEC) [43].
Thermosensitive polymers in aqueous solutions display temperature-dependent sol to gel transitions at specified levels. If the solidification appears above a certain temperature, the temperature is referred to as the lower critical solution temperature (LCST). Corresponding polymers are soluble below the LCST, even if solubility decreases above the LCST due to increased hydrophobicity. This contributes in a reversible gelation of the solution. In the drug delivery, the LCST should be between the average ambient temperature (25 °C) and the body temperature (37 °C). In this manner, the drug can be applied as a liquid and then transferred to gel after administration [54][55][56]. In the case of formulations showing sol-gel transition upon cooling one speaks of the upper critical solution temperature (UCST) [56] [57][58]. Most natural polymers in aqueous solutions undergo sol-gel transformation at lower temperatures. However, some cellulose derivatives gel at higher temperatures. As the temperature increases, they gradually lose water, increasing intermolecular interactions, which are primarily mediated by methoxy moieties. As a result, the network structure is formed, which corresponds to the system gelation. The LCSTs of MC and HPMC are between 40 to 50 °C and 75 to 90 °C, respectively [26], but chemical or physical modification may result in higher desired values [56][59][60].
Cellulose ethers (CEs) are other bioadhesive polymers. Cellulose is a linear homopolymer polysaccharide consisting of D-anhydroglucopyranose units joined together by ß-1,4-glycosidic bonds [61]. Cellulose is insoluble in water due to extensive intramolecular hydrogen bonding. Thermogelation of cellulose derivatives varies with the degree and type of substitution. CEs are produced by etherification of the three hydroxyl groups of cellulose anhydroglucose components, which produce water-soluble derivatives. The results are the production of CEs such as MC, HPMC and CMC [62]. CMC is an ionic ether of cellulose and it is the major commercial derivative in which original H atoms of cellulose hydroxyl groups are replaced by carboxymethyl substituent [63]. However, MC and HPMC are used frequently. MC is a cellulose derivative that has been widely studied for biomedical applications. It is long-chain substituted cellulose consisting approximately 27–32% of the hydroxyl groups in the methyl ether form [61][63]. It has thermoreversible gelation properties in aqueous solutions and gells at temperatures between 60 and 80 °C and transforms again into solution at lower temperature [61][64][65]. HPMC is partly O-methylated and O-(2-hydroxypropylated) cellulose [63]. Methoxy residues of HPMC are responsible for the gelation, due to the increase in hydrophobic interactions and exclusion of water from heavily methoxylated regions of the polymer [61][66].
Sodium alginate (Alg-Na) is a naturally occurring mucoadhesive biopolymer (Figure 2d). This polysaccharide is obtained mainly from brown algae belonging to the Phaeophyceae and composed of α–L-guluronic acid and β-d-mannuronic acid residues. Alginate has several desirable properties, such as biodegradability, non-toxicity, biocompatibility, and low cost, making it a promising biopolymer for various applications in DDSs [67]. PVP is one of the most widely used polymers in medicine due to its solubility in water and its extremely low cytotoxicity (Figure 2b). Another advantage of using PVP is that it can be thermally crosslinked, resulting in outstanding thermal stability and high mechanical strength of the material [68][69]. Polyacrylates, such as carbopol and PCP, are the most effective in mucoadhesive action since they have a high molecular mass (Figure 2e). This feature offers a relatively high period of residence on the mucosa [49][70]. Carbopol has 58–68% of carboxylic groups which progressively undergo hydrogen bonding with sugar residues in oligosaccharide chains in the mucus membrane [71]. It results in the formation of a strengthened network between polymer and mucus membrane, so that carbopol having a high density of available hydrogen bonding groups is able to interact more strongly with mucin glycoproteins. It is speculated that enhanced mucoadhesive strength of the delivery system may lead to prolonged retention and increased absorption of drugs across mucosal tissues [71][72]. Carbopol, as a synthetic polymer, has been often used as a component of RDDSs. Due to its high viscosity, it may be used as the bioadhesive polymer to reinforce the gel strength of the P407/P188 thermosensitive hydrogel [53][73].
The addition of non-ionic surfactants is also often used in the manufacture of thermosensitive liquid suppositories. These compounds can act as wetting agents and can have a positive impact on the drugs release [74]. The addition of Tween (most commonly Tween 80) results in highly viscous gel formation, increased mucoadhesive strength and decreased gelation temperature and time. The mechanism by which Tween 80 influences the properties of the gel can be the reinforcement of the hydrogen bonding between the poloxamer combination in the gel matrix [75][76]. Non-ionic surfactants such as Tween 80 have been reported to be inert, resulting in no damage to mucous membranes [28]. In rectal administration, Tween 80 does not reveal any side effects.
In order to be used for treatment, the thermosensitive liquid suppository must have satisfactory rheological and mechanical properties, such as:
- gelation temperature: the temperature at which the liquid phase is transformed to the gel phase. The gelation temperature range that would be appropriate for rectal administration is 30–36.5 °C;
- viscosity: viscosity of the thermosensitive liquid suppository at 36.5 °C is known as gel strength; liquid suppository with optimal gel strength (10–50 s) will remain in the upper part of the rectum and will not leak out from the anus;
- gelation time and gel strength: thermosensitive liquid suppository with a relatively faster gelation time and optimal gel strength will remain in the upper part of the rectum and will not leak out from the anus. Gelation time means the time taken for the thermosensitive liquid suppository to achieve a viscosity of approximately 4000 mPa·s at 36.5 °C. Gelation time varies according to suppository composition, but is usually 2–8 min;
- mucoadhesive force: the force by which the thermosensitive liquid suppository binds to the mucous membranes of the rectal.
Reasonable rheological and mechanical properties can be obtained by using the different proportions of mucoadhesive polymers referred to above [38][77].
The most popular method of obtaining thermosensitive liquid suppository is cold method [78]. The mucoadhesive polymers can be used at different concentrations, as discussed later in this paper.
2. Application of Thermosensitive Liquid Suppositories as Innovative Systems for Delivering Various Drugs
One of the key advancements in modern pharmacology is the development of new drugs, new drug formulations or new DDSs. These solutions enable the delivery of active substances in a particular place, at the right time, using the most preferred form of administration, and with minimal side effects. The discovery of new synthetic drugs is, however, time consuming and expensive—thus, modern pharmaceutical science is generally focused on improving the pharmacokinetics of known drugs or developing innovative drug dosage forms. The production of thermosensitive liquid suppositories is one example. This dosage method modifies the pharmacokinetic properties of drugs and retains all the benefits of the rectal route. The available reports contain studies on thermosensitive liquid suppositories that deliver a range of drugs, e.g., analgesic, anticancer, antihypertensive, anaesthetic, antiemetic, antimalarial, antiallergic psychiatric and insulin.
References
- Verma, P.; Thakur, A.S.; Deshmukh, K.; Jha, D.A.K.; Verma, S.; Routes of drug administration. Int. J. Pharm. Sci. Res. 2010, 1, 54-59.
- Preshita Desai; Abhijit Date; Vandana B. Patravale; Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations. Drug Discovery Today: Technologies 2012, 9, e87-e95, 10.1016/j.ddtec.2011.12.001.
- G. Dumortier; J. L. Grossiord; M. Zuber; G. Couarraze; J. C. Chaumeil; Rheological study of a thermoreversible morphine gel. Drug Development and Industrial Pharmacy 1991, 17, 1255-1265, 10.3109/03639049109043858.
- Matthias Klueglich; Arne Ring; Stefan Scheuerer; Dirk Trommeshauser; Chris Schuijt; Bernd Liepold; Gunther Berndl; Ibuprofen Extrudate, a Novel, Rapidly Dissolving Ibuprofen Formulation: Relative Bioavailability Compared to Ibuprofen Lysinate and Regular Ibuprofen, and Food Effect on All Formulations. The Journal of Clinical Pharmacology 2005, 45, 1055-1061, 10.1177/0091270005279579.
- Marilyn N. Martinez; Gordon L. Amidon; A Mechanistic Approach to Understanding the Factors Affecting Drug Absorption: A Review of Fundamentals. The Journal of Clinical Pharmacology 2002, 42, 620-643, 10.1177/00970002042006005.
- Vinod P. Shah; Gordon L. Amidon; G.L. Amidon, H. Lennernas, V.P. Shah, and J.R. Crison. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of In Vitro Drug Product Dissolution and In Vivo Bioavailability, Pharm Res 12, 413–420, 1995—Backstory of BCS. The AAPS Journal 2014, 16, 894-898, 10.1208/s12248-014-9620-9.
- Mohammad-Ali Shahbazi; Hélder A Santos; Improving oral absorption via drug-loaded nanocarriers: absorption mechanisms, intestinal models and rational fabrication.. Current Drug Metabolism 2013, 14, 28-56.
- Michael Goldberg; Isabel Gomez-Orellana; Challenges for the oral delivery of macromolecules. Nature Reviews Drug Discovery 2003, 2, 289-295, 10.1038/nrd1067.
- Farnaz Esmaeili; Rassoul Dinarvand; Mohammad Hossein Ghahremani; Mohsen Amini; Hasti Rouhani; Nima Sepehri; Seyed Nasser Ostad; Fatemeh Atyabi; Docetaxel–Albumin Conjugates: Preparation, In Vitro Evaluation and Biodistribution Studies. Journal of Pharmaceutical Sciences 2009, 98, 2718-2730, 10.1002/jps.21599.
- Robert M. Straubinger; Sathyamangalam V. Balasubramanian; Preparation and Characterization of Taxane-Containing Liposomes. Methods in Enzymology 2005, 391, 97-117, 10.1016/s0076-6879(05)91005-7.
- Nahla S. Barakat; In Vitro and In Vivo Characteristics of a Thermogelling Rectal Delivery System of Etodolac. AAPS PharmSciTech 2009, 10, 724-731, 10.1208/s12249-009-9261-y.
- Makrem Ben Reguiga; Laurence Bonhomme-Faivre; Robert Farinotti; Bioavailability and tissular distribution of docetaxel, a P-glycoprotein substrate, are modified by interferon-α in rats. Journal of Pharmacy and Pharmacology 2007, 59, 401-408, 10.1211/jpp.59.3.0010.
- Farnaz Esmaeili; Rassoul Dinarvand; Mohammad Hossein Ghahremani; Seyed Nasser Ostad; Hadi Esmaily; Fatemeh Atyabi; Cellular cytotoxicity and in-vivo biodistribution of docetaxel poly(lactide-co-glycolide) nanoparticles. Anti-Cancer Drugs 2010, 21, 43-52, 10.1097/cad.0b013e328331f934.
- Vincent Jannin; Gilles Lemagnen; Pascale Gueroult; Denis Larrouture; Catherine Tuleu; Rectal route in the 21st Century to treat children. Advanced Drug Delivery Reviews 2014, 73, 34-49, 10.1016/j.addr.2014.05.012.
- Larry Caldwell; Toshiaki Nishihata; J. Howard Rytting; Takeru Higuchi; Lymphatic uptake of water-soluble drugs after rectal administration. Journal of Pharmacy and Pharmacology 1982, 34, 520-522, 10.1111/j.2042-7158.1982.tb04778.x.
- Trusha J. Purohit; Sara M. Hanning; Zimei Wu; Advances in rectal drug delivery systems. Pharmaceutical Development and Technology 2018, 23, 942-952, 10.1080/10837450.2018.1484766.
- Gray, H.; Williams, P.L; Banister, L.H.. Gray’s Anatomy, 38th ed.; Churchill Livingstone: Edinburgh: New York, NY, USA, 1995; pp. 1240-1243.
- Bar-Shalom, D.; Klaus, R.. Pediatric Formulations: A Roadmap; Springer: New York, USA, 2014; pp. 303-310.
- Lakshmi, J.P.; Deepthi, B.; Rama, R.N.; Rectal Drug Delivery: A promising route for enhancing drug absorption.. Asian J. Pharm. Sci. 2012, 2, 143-149.
- De Boer, A.G.; Rate controlled rectal peptide absorption enhancement. In penetration enhancement for polypeptides through epithelia.. Adv. Drug Deliver. Rev. 2012, 2, 143-149.
- F Moolenaar; J P Yska; J Visser; D K Meijer; Drastic improvement in the rectal absorption profile of morphine in man.. European Journal of Clinical Pharmacology 1985, 29, 119-121.
- Vijay D. Havaldar; Adhikrao Yadav; Remeth Dias; Kailas Mali; Vishwajeet Ghorpade; Nitin H. Salunkhe; Rectal suppository as an effective alternative for oral administration. Research Journal of Pharmacy and Technology 2015, 8, 759, 10.5958/0974-360x.2015.00122.5.
- Baviskar, P.; Bedse, A.; Sadique, S.; Kunde, V.; Jaiswal, S.; Drug delivery on rectal absorption: Suppositories.. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 70-76.
- Jain, N.K.. Progress in Controlled & Novel Drug Delivery Systems; CBS Publisher & Distributors: New Delhi, India, 2010; pp. 209-247.
- Ewoud J. Van Hoogdalem; Albertus G. De Boer; Douwe D. Breimer; Pharmacokinetics of Rectal Drug Administration, Part I. Clinical Pharmacokinetics 1991, 21, 11-26, 10.2165/00003088-199121010-00002.
- Ewoud J. Van Hoogdalem; Ewoud J. Van Hoogdalem; Albertus G. De Boer; Douwe D. Breimer; Pharmacokinetics of Rectal Drug Administration, Part II. Clinical Pharmacokinetics 1991, 21, 110-128, 10.2165/00003088-199121020-00003.
- Ehab Ahmed Hosny; Hasan Ibrahim Al-Shora; Mohamad Mohey Aldin Elmazar; Relative Hypoglycemic Effect of Insulin Suppositories in Diabetic Beagle Dogs: Optimization of Various Concentrations of Sodium Salicylate and Polyoxyethylene-9-lauryl Ether.. Biological and Pharmaceutical Bulletin 2001, 24, 1294-1297, 10.1248/bpb.24.1294.
- M Yun; Development of a thermo-reversible insulin liquid suppository with bioavailability enhancement. International Journal of Pharmaceutics 1999, 189, 137-145, 10.1016/s0378-5173(99)00227-6.
- Elissa Bradshaw; Brigitte Collins; Julia Williams; Administering rectal suppositories: Preparation, assessment and insertion. Gastrointestinal Nursing 2009, 7, 24-28, 10.12968/gasn.2009.7.9.45271.
- Ramadan, E.M.; Borg, T.M.; Elkayal, M.O.; Formulation and evaluation of novel mucoadhesive ketorolac tromethamine liquid suppository. AJPP 2009, 3, 124-132.
- Özgüney, I.; Kardhiqi, A.; Yıldız, G.; Ertan, G.; In vitro–in vivo evaluation of In Situ gelling and thermosensitive ketoprofen liquid suppositories. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 283-291.
- Rectal and Vaginal Drug Delivery . Rectal and Vaginal Drug Delivery. Retrieved 2021-7-28
- How to Use Rectal Suppositories Properly. American Society of Health-System Pharmacists . How to Use Rectal Suppositories Properly. American Society of Health-System Pharmacists. Retrieved 2021-7-28
- Jones, D.S. Pharmaceutics—Dosage Form and Design; Pharmaceutical Press: London, UK, 2008; pp. 218.
- Toshiaki Nishihata; Junko Kato; Kiheun Kim; Motomasa Kobayashi; Isao Kitagawa; Akira Kamada; Formation and hydrolysis of enamine in aqueous solution.. Chemical and Pharmaceutical Bulletin 1984, 32, 4545-4550, 10.1248/cpb.32.4545.
- Chih, H.C.; Tadakazu, T.; Yoshiharu, M.; Tsuneji, N.; Formulation of double-layered suppository for prolonged stay in lower rectum.. JPST 1987, 47, 42-48.
- Han-Gon Choi; Mi-Kyung Lee; Moon-Hee Kim; Chong-Kook Kim; Effect of additives on the physicochemical properties of liquid suppository bases. International Journal of Pharmaceutics 1999, 190, 13-19, 10.1016/s0378-5173(99)00225-2.
- Yong Chul Soon; Choi Young-Kwon; Chul Soon Yong; Park Byung-Joo; Quan Qi-Zhe; Rhee Jong-Dai; Kim Chong-Kook; Choi Han-Gon; Physicochemical characterization andin vivo evaluation of thermosensitive diclofenac liquid suppository. Archives of Pharmacal Research 2003, 26, 162-167, 10.1007/bf02976664.
- Helen Trenerry; The complete drug reference. 37th ed. Australian Prescriber 2011, 34, 195, 10.18773/austprescr.2011.099.
- Özgüney, I.; Kardhiqi, A.; Properties of bioadhesive ketoprofen liquid suppositories: Preparation, determination of gelation temperature, viscosity studies and evaluation of mechanical properties using texture analyzer by 4 × 4 factorial design.. Pharm. Dev. Technol 2013, 19, 968-975.
- E. Pasztor; A. Makó; G. Csóka; Zs. Fenyvesi; R. Benko; M. Prosszer; S. Marton; I. Antal; I. Klebovich; New formulation of in situ gelling Metolose-based liquid suppository. Drug Development and Industrial Pharmacy 2010, 37, 1-7, 10.3109/03639045.2010.489558.
- Jin Whan Lee; Jae Han Park; Joseph R. Robinson; Bioadhesive‐based dosage forms: The next generation. Journal of Pharmaceutical Sciences 2000, 89, 850-866, 10.1002/1520-6017(200007)89:7<850::aid-jps2>3.3.co;2-7.
- A.A. Koffi; F. Agnely; G. Ponchel; J.L. Grossiord; Modulation of the rheological and mucoadhesive properties of thermosensitive poloxamer-based hydrogels intended for the rectal administration of quinine. European Journal of Pharmaceutical Sciences 2006, 27, 328-335, 10.1016/j.ejps.2005.11.001.
- Youn Gee Seo; Dong-Wuk Kim; Woo Hyun Yeo; Thiruganesh Ramasamy; Yu-Kyoung Oh; Young-Joon Park; Jung-Ae Kim; Dong Hoon Oh; Sae Kwang Ku; Jin Ki Kim; et al.Chul Soon YongJong Oh KimHan-Gon Choi Docetaxel-Loaded Thermosensitive and Bioadhesive Nanomicelles as a Rectal Drug Delivery System for Enhanced Chemotherapeutic Effect. Pharmaceutical Research 2013, 30, 1860-1870, 10.1007/s11095-013-1029-0.
- Jong Oh Kim; Dong-Wuk Kim; Thiruganesh Ramasamy; Ju Yeon Choi; Jeong Hwan Kim; Chul Soon Yong; Han-Gon Choi; The influence of bile salt on the chemotherapeutic response of docetaxel-loaded thermosensitive nanomicelles. International Journal of Nanomedicine 2014, 9, 3815-3824, 10.2147/ijn.s64794.
- Kassab, H.J.; Khalil, Y.I.; 5-Fluorouracil mucoadhesive liquid formulation and evaluation.. World J. Pharm. Res. 2014, 3, 119-135.
- Sagrado, F.G.; Guzman, M.; Molpeceres, J.; Aberturas, M.; Pluronic copolymer—characteristics, properties and pharmaceutical applications: Part I.. Pharm. Technol. Eur. 1994, 6, 46-56.
- Jose Juan Escobar-Chavez; M López-Cervantes; A Naïk; Yogeshvar Kalia; D Quintanar-Guerrero; A Ganem-Quintanar; Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations.. Journal of Pharmacy & Pharmaceutical Sciences 2006, 9, 339-358.
- Henry, R.L.; Burn wound coverings and the use of poloxamer preparations. Crit. Rev. Biocompat. 1989, 5, 207-220.
- Mariko Morishita; Jose Mario Barichello; Kozo Takayama; Yoshiyuki Chiba; Shinji Tokiwa; Tsuneji Nagai; Pluronic® F-127 gels incorporating highly purified unsaturated fatty acids for buccal delivery of insulin. International Journal of Pharmaceutics 2001, 212, 289-293, 10.1016/s0378-5173(00)00615-3.
- Katarina Edsman; Johan Carlfors; Roger Petersson; Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. European Journal of Pharmaceutical Sciences 1998, 6, 105-112, 10.1016/s0928-0987(97)00075-4.
- Jianping Chen; Rong Zhou; Lin Li; Bing Li; Xia Zhang; Jianyu Su; Mechanical, Rheological and Release Behaviors of a Poloxamer 407/ Poloxamer 188/Carbopol 940 Thermosensitive Composite Hydrogel. Molecules 2013, 18, 12415-12425, 10.3390/molecules181012415.
- Gang Wei; Hui Xu; Ping Tian Ding; San Ming Li; Jun Min Zheng; Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. Journal of Controlled Release 2002, 83, 65-74, 10.1016/s0168-3659(02)00175-x.
- Eve Ruel-Gariépy; Jean-Christophe Leroux; In situ-forming hydrogels?review of temperature-sensitive systems. European Journal of Pharmaceutics and Biopharmaceutics 2004, 58, 409-426, 10.1016/s0939-6411(04)00087-6.
- Laura Mayol; Marco Biondi; Fabiana Quaglia; Sabato Fusco; Assunta Borzacchiello; Luigi Ambrosio; Maria I. La Rotonda; Injectable Thermally Responsive Mucoadhesive Gel for Sustained Protein Delivery. Biomacromolecules 2011, 12, 28-33, 10.1021/bm1008958.
- Forouhe Zahir-Jouzdani; Julian Dominik Wolf; Fatemeh Atyabi; Andreas Bernkop-Schnürch; In situ gelling and mucoadhesive polymers: why do they need each other?. Expert Opinion on Drug Delivery 2018, 15, 1007-1019, 10.1080/17425247.2018.1517741.
- Klouda, L.; Mikos, A.G.; Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34-45.
- Sing Shy Liow; Qingqing Dou; Dan Kai; Anis Abdul Karim; Kangyi Zhang; Fujian Xu; Xian Jun Loh; Thermogels: In Situ Gelling Biomaterial. ACS Biomaterials Science & Engineering 2016, 2, 295-316, 10.1021/acsbiomaterials.5b00515.
- Hanna Park; Min Hee Kim; Young Il Yoon; Won Ho Park; One-pot synthesis of injectable methylcellulose hydrogel containing calcium phosphate nanoparticles. Carbohydrate Polymers 2017, 157, 775-783, 10.1016/j.carbpol.2016.10.055.
- N. Sarkar; Thermal gelation properties of methyl and hydroxypropyl methylcellulose. Journal of Polymer Science 1979, 24, 1073-1087, 10.1002/app.1979.070240420.
- Jain, S.; Sandhu, P.S.; Malvi, R.; Gupta, B.; Cellulose derivatives as thermoresponsive polymer: An overview.. J. Appl. Pharm. Sci. 2013, 3, 139-144.
- Shaoyu Lü; Mingzhu Liu; Boli Ni; Chunmei Gao; A novel pH- and thermo-sensitive PVP/CMC semi-IPN hydrogel: Swelling, phase behavior, and drug release study. Journal of Polymer Science Part B: Polymer Physics 2010, 48, 1749-1756, 10.1002/polb.22040.
- Rowe, R.C. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, 2009; pp. 129.
- L. Li; † H. Shan; † C. Y. Yue; Yee Cheong Lam; Michael Kc Tam; Xiao Hu; Thermally Induced Association and Dissociation of Methylcellulose in Aqueous Solutions. Langmuir 2002, 18, 7291-7298, 10.1021/la020029b.
- Takahashi, M.; Shimazaki, M.; Yamamoto, J.; Thermoreversible gelation and phase separation in aqueous methyl aellulose solutions. J. Polym. Sci. B Polym. Phys. 2001, 39, 91-100.
- James L. Ford; Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: powders, gels and matrix tablets. International Journal of Pharmaceutics 1999, 179, 209-228, 10.1016/s0378-5173(98)00339-1.
- Zhao-Hong Hu; Ahmed Mohamed Omer; Xiao–Kun Ouyang; Di Yu; Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. International Journal of Biological Macromolecules 2018, 108, 149-157, 10.1016/j.ijbiomac.2017.11.171.
- Haijun Yu; Xiaoyi Xu; Xuesi Chen; Tiancheng Lu; Peibiao Zhang; Xiabin Jing; Preparation and antibacterial effects of PVA-PVP hydrogels containing silver nanoparticles. Journal of Polymer Science 2006, 103, 125-133, 10.1002/app.24835.
- Maoping Zheng; Mingyuan Gu; Yanping Jin; Guoliang Jin; Preparation, structure and properties of TiO2–PVP hybrid films. Materials Science and Engineering: B 2000, 77, 55-59, 10.1016/s0921-5107(00)00465-7.
- Asane, G.S.; Nirmal, S.A.; Rasal, K.B.; Naik, A.A.; Mahadik, M.S.; Rao, Y.M.; Polymers for Mucoadhesive Drug Delivery System: A Current Status.. Drug Dev. Ind. Pharm. 2008, 34, 1246–1266.
- Jun Kunisawa; Akiko Okudaira; Yasuo Tsutusmi; Ichiro Takahashi; Tsuyoshi Nakanishi; Hiroshi Kiyono; Tadanori Mayumi; Characterization of mucoadhesive microspheres for the induction of mucosal and systemic immune responses.. Vaccine 2000, 19, 589-594, 10.1016/s0264-410x(00)00094-3.
- Rita J. Majithiya; Pradip K. Ghosh; Manish L. Umrethia; Rayasa S. R. Murthy; Thermoreversible-mucoadhesive Gel for nasal delivery of sumatriptan. AAPS PharmSciTech 2006, 7, E80-E86, 10.1208/pt070367.
- Rathapon A-Sasutjarit; Anuvat Sirivat; Panida Vayumhasuwan; Viscoelastic Properties of Carbopol 940 Gels and Their Relationships to Piroxicam Diffusion Coefficients in Gel Bases. Pharmaceutical Research 2005, 22, 2134-2140, 10.1007/s11095-005-8244-2.
- J.E. Fontan; P. Arnaud; J.C. Chaumeil; Enhancing properties of surfactants on the release of carbamazepine from suppositories. International Journal of Pharmaceutics 1991, 73, 17-21, 10.1016/0378-5173(91)90095-6.
- Jalal Hanaee; Yousef Javadzadeh; S. Taftachi; D. Farid; A. Nokhodchi; The role of various surfactants on the release of salbutamol from suppositories. Il Farmaco 2004, 59, 903-906, 10.1016/j.farmac.2004.07.006.
- Fakhar- Ud- Din; Gul Majid Khan; Development and characterisation of levosulpiride-loaded suppositories with improved bioavailability in vivo. Pharmaceutical Development and Technology 2017, 24, 63-69, 10.1080/10837450.2017.1419256.
- Polly E Kintzel; Laura B Michaud; Marianne K Lange; Docetaxel-Associated Epiphora. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2006, 26, 853-867, 10.1592/phco.26.6.853.
- Mandar J. Bhandwalkar; Amelia M. Avachat; Thermoreversible Nasal In situ Gel of Venlafaxine Hydrochloride: Formulation, Characterization, and Pharmacodynamic Evaluation. AAPS PharmSciTech 2012, 14, 101-110, 10.1208/s12249-012-9893-1.




