Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SEUNGEUN LEE | + 1278 word(s) | 1278 | 2021-05-16 18:03:56 | | | |
| 2 | Dean Liu | Meta information modification | 1278 | 2021-07-27 08:49:59 | | | | |
| 3 | Dean Liu | -2 word(s) | 1276 | 2021-07-28 10:05:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lee, S. Transduodenal Ampullectomy. Encyclopedia. Available online: https://encyclopedia.pub/entry/12481 (accessed on 07 February 2026).
Lee S. Transduodenal Ampullectomy. Encyclopedia. Available at: https://encyclopedia.pub/entry/12481. Accessed February 07, 2026.
Lee, Seungeun. "Transduodenal Ampullectomy" Encyclopedia, https://encyclopedia.pub/entry/12481 (accessed February 07, 2026).
Lee, S. (2021, July 27). Transduodenal Ampullectomy. In Encyclopedia. https://encyclopedia.pub/entry/12481
Lee, Seungeun. "Transduodenal Ampullectomy." Encyclopedia. Web. 27 July, 2021.
Copy Citation
Transduodenal ampullectomy (TDA) has lower peri-operative morbidity and mortality than PD and has been suggested as an alternative surgical treatment for ampullary adenoma and early AoV cancer.
ampulla of Vater cancer
transduodenal ampullectomy
pancreaticoduodenectomy
1. Introduction
Ampulla of Vater (AoV) cancer is a malignant neoplasm that develops from the ampulla of Vater complex, which lies between the area distal to the confluence of the pancreatic duct and the distal common bile duct (CBD) and the duodenal opening. AoV cancer is a rare disease that constitutes roughly 7% of peri-ampullary tumors [1][2]. Although AoV cancer is a peri-ampullary tumor, it has a better prognosis than other peri-ampullary tumors such as pancreatic cancer and distal CBD cancer [3][4][5]. Approximately half of patients with AoV cancer present at an advanced stage. For those who present at an early stage, pancreaticoduodenectomy (PD) or pylorus-preserving pancreaticoduodenectomy (PPPD) are recognized as standard treatments [6][7][8]. Because the disease generally presents in patients whose age makes surgical procedures risky, only 40% of AoV patients undergo surgical resection [9].
Transduodenal ampullectomy (TDA) has lower peri-operative morbidity and mortality than PD and has been suggested as an alternative surgical treatment for ampullary adenoma and early AoV cancer [10][11][12]. However, TDA presents a higher chance of recurrence due to limited resection and insufficient lymph node dissection, and its oncological safety has been controversial [13]. Therefore, TDA is used only in patients with high surgical co-morbidity factors, small cancer with good cell differentiation, and staging equivalent to or less than T1. The indications for TDA differ among institutes, and no clear guidelines have been established [14][15]. Some previous studies have compared the oncologic outcomes of PD and TDA in early AoV cancer. Winter et al. from the Johns Hopkins group reported no statistical differences (p = 0.150) in postoperative complications between PD (n = 435) and TDA (n = 15). They noticed a high incidence of lymph node metastasis (28.0%) in T1 cancer and concluded that PD should be preferred, even in early AoV cancer [16]. A retrospective single-institution study of early AoV cancer in China (PD: n = 21, TDA: n = 22) reported no statistical differences between the two groups in 5-year survival outcomes and a lower complication rate in the TDA group [17]. A retrospective single-institution study in Korea evaluated the clinicopathologic characteristics, disease-free survival (DFS), and recurrence rate in 137 patients (PD: n = 119, TDA: n = 18) with early AoV cancer (Tis or T1). Those authors reported no statistical differences in postoperative complications or DFS between the two groups; however, among patients with T1 cancer, the TDA group showed a statistically higher recurrence rate than the PD group [18].
2. Clinicopathologic Characteristics of the PPPD, TDA+LND, and TDA-Only Groups with T1 Stage Disease
Because patients with T1 disease had better DFS following PPPD than TDA (84.8% vs. 66.6%, p = 0.040), we analyzed differences in the clinicopathologic characteristics of the T1 stage patients in both groups. For this analysis, we divided surgical methods into three groups: PPPD, TDA+LND, and TDA-only. The PPPD and TDA+LND groups did not differ in age, sex, tumor size, N stage, cell differentiation, LVI, PNI, or adjuvant treatment. However, the R0 resection rate was higher in the PPPD group than in the TDA+LND group (100% vs. 84.6%, p = 0.004). In the comparison between the PPPD and TDA-only groups, the tumors were significantly larger (1.8 vs. 1.2 cm, p = 0.025), and cell differentiation was worse in the PPPD group than in the TDA-only group (p = 0.008) (Table 1).
Table 1. Comparison of clinicopathologic characteristics and recurrence according to the operation type in patients with T1 stage disease.
| Operation | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 1. PPPD (188) | 2. TDA + LND (13) | 3. TDA-Only (18) | 1 vs. 2 | 1 vs. 3 | 2 vs. 3 | Total | |
| Age (years) | 63.07 ± 9.03 | 62.15 ± 11.66 | 59.39 ± 16.17 | 0.729 | 0.354 | 0.584 | 0.319 |
| Male (%) | 86 (45.7%) | 6 (46.2%) | 9 (50.0%) | 0.977 | 0.729 | 0.833 | 0.942 |
| Size (cm) | 1.81 ± 1.09 | 1.76 ± 0.68 | 1.20 ± 0.79 | 0.866 | 0.025 | 0.049 | 0.073 |
| N staging | |||||||
| Nx | 6 (3.2%) | 18 (100.0%) | 1.000 | <0.001 | <0.001 | <0.001 | |
| N0 | 161 (85.6%) | 12 (92.3%) | |||||
| N1 | 21 (11.2%) | 1 (7.7%) | |||||
| Differentiation | |||||||
| Well | 113 (62.1%) | 8 (72.7%) | 14 (82.4%) | 0.845 | 0.008 | 0.253 | 0.017 |
| Moderate | 61 (33.5%) | 3 (27.3%) | 1 (5.9%) | ||||
| Poorly | 6 (3.3%) | 0 (0.0%) | 0 (0.0%) | ||||
| Undiff. | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Etc. | 2 (1.1%) | 0 (0.0%) | 2 (11.8%) | ||||
| LVI (+) | 35 (22.9%) | 0 (0.0%) | 0 (0.0%) | 0.349 | 0.576 | − | 0.202 |
| PNI (+) | 22 (14.4%) | 0 (0.0%) | 0 (0.0%) | 0.595 | 1.000 | − | 0.340 |
| Adj. Tx | 18 (9.6%) | 1 (7.7%) | 0 (0.0%) | 1.000 | 0.377 | 0.419 | 0.383 |
| R status | |||||||
| R0 | 188 (100.0%) | 11 (84.6%) | 18 (100.0%) | 0.004 | − | 0.168 | <0.001 |
| R1 | 0 (0.0%) | 2 (15.4%) | 0 (0.0%) | ||||
| Recurrence | 20 (10.6%) | 3 (23.1%) | 5 (27.8%) | 0.174 | 0.050 | 1.000 | 0.019 |
| Recurrence pattern | 0.249 | 0.016 | 1.000 | 0.004 | |||
| Local | 1 (0.5%) | 1 (7.7%) | 3 (16.7%) | ||||
| Systemic | 19 (10.1%) | 2 (15.4%) | 2 (11.1%) | ||||
PPPD: pylorus preserving pancreaticoduodenectomy, TDA: transduodenal ampullectomy, Undiff.: undifferentiated, LVI: lymphovascular invasion, PNI: perineural invasion, adj. Tx: adjuvant treatment.
3. Comparison of Survival Outcomes
We analyzed the 5-year DFS and OS rates by dividing the study population into three groups by T stage, and we compared the PPPD and TDA groups using the entire population (Tis, T1, and T2), Tis + T1, and T1-only. We also performed survival analysis by dividing the TDA group into TDA+LND and TDA-only groups. In the entire study population (Tis, T1, and T2), the overall recurrence rate did not differ between the PPPD and TDA groups (p = 0.258). However, the local recurrence rate was higher in the TDA group than in the PPPD group (10.3% vs. 5.5%, p = 0.012), and the systemic recurrence rate was higher in the PPPD group (16.7% vs. 5.9%, p = 0.012). Within the systemic recurrences, peritoneal seeding was observed in 7 patients (1.7%) in the PPPD group and 1 patient (1.5%) in the TDA group, which was without statistical difference (p = 1.000) (Table 2). The 5-year DFS and OS rates did not differ between the PPPD and TDA groups (71.9% vs. 78.6%, p = 0.156, 74.2% vs. 77.6%, p = 0.816, respectively, Figure 2A,B). When survival outcomes were analyzed among the PPPD, TDA+LND, and TDA-only groups, neither DFS (PPPD vs. TDA+LND vs. TDA-only, 71.9% vs. 79.3% vs. 78.1%, p = 0.366) nor OS (74.2% vs. 82.6% vs. 75.6%, p = 0.932) differed statistically (Figure 2C,D).
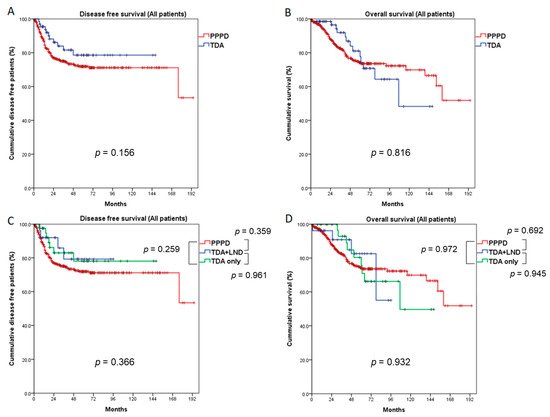
Figure 2. Comparison of survival outcomes between the PPPD and TDA groups (all patients: Tis, T1, and T2). Five-year disease-free survival (A) and overall survival (B) did not differ between the PPPD and TDA groups. When the TDA group was divided into TDA + LND and TDA-only groups, 5-year disease-free survival (C) and overall survival (D) did not differ among the three groups. LND: lymph node dissection.
In the Tis + T1 study population, 5-year DFS and OS did not differ statistically between the PPPD and TDA groups (84.5% vs. 80.1%, p = 0.596, 88.4% vs. 76.0%, p = 0.122, respectively, Figure 3A,B), though local recurrence was more frequently observed in the TDA group (7.3% vs. 1.0%, p = 0.033). Even when comparing the PPPD, TDA+LND and TDA-only groups, DFS (p = 0.868) and OS (p = 0.286) did not differ (Figure 3C,D).
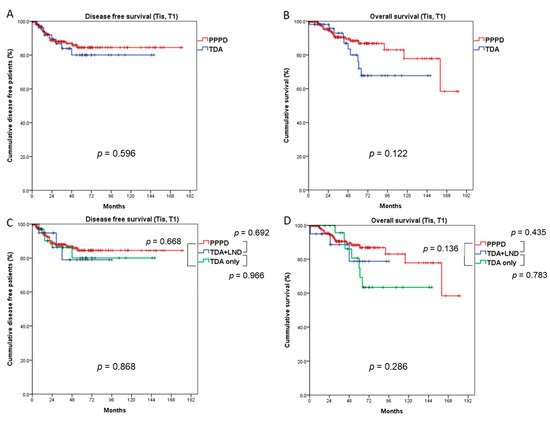
Figure 3. Comparison of survival outcomes between the PPPD and TDA groups (Tis and T1 patients). Five-year disease-free survival (A) and overall survival (B) did not differ between the PPPD and TDA groups. When the TDA group was divided into TDA + LND and TDA-only groups, the 5-year disease-free survival (C) and overall survival (D) did not differ among the three groups. LND: lymph node dissection.
In the T1 population, 5-year DFS was significantly better in the PPPD group than in the TDA group (84.8% vs. 66.6%, p = 0.040), and 5-year OS was marginally better in the PPPD group than the TDA group (89.1% vs. 68.0%, p = 0.056) (Figure 4A,B). When the TDA group was divided into TDA+LND and TDA-only groups, the PPPD group showed a better 5-year DFS rate than the TDA-only group (84.8% vs. 64.1%, p = 0.046). Although there was no statistical significance, 5-year DFS in the PPPD group was also better than that in the TDA+LND group (84.8% vs. 68.8%, p = 0.241). Five-year OS did not differ significantly among the three groups (PPPD vs. TDA+LND, 89.1% vs. 69.2%, p = 0.138; PPPD vs. TDA-only, 89.1% vs. 67.1%, p = 0.138; TDA+LND vs. TDA-only, 69.2% vs. 67.1%, p = 0.870); however, the PPPD group had a better prognosis by more than 20% compared with the TDA+LND and TDA-only groups. (Figure 4C,D). When the recurrence pattern was analyzed, local recurrence occurred significantly more often in the TDA-only group than in the PPPD group (16.7% vs. 0.5%, p = 0.016).
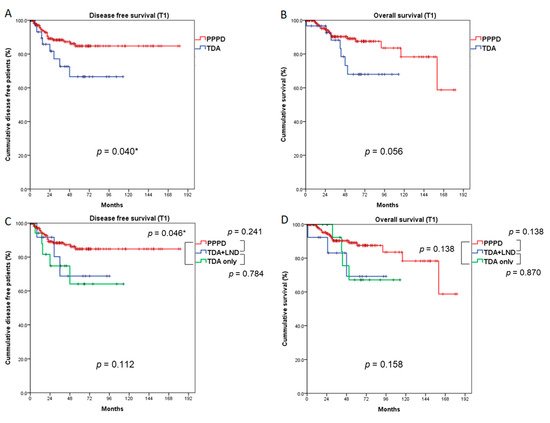
Figure 4. Comparison of survival outcomes between the PPPD and TDA groups (T1 patients). Five-year disease-free survival (A) was significantly better after PPPD than TDA (p = 0.040). Five-year overall survival (B) was marginally better in the PPPD group than the TDA group (p = 0.056). When the TDA group was divided into TDA + LND and TDA-only groups, the PPPD group showed better disease-free survival than the TDA-only group (p = 0.046), but disease-free survival did not differ between the PPPD and TDA+LND groups (C). Five-year overall survival (D) did not differ among the three groups. LND: lymph node dissection. * statistically significant (p < 0.050)
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96.
- Howard, J.M.; Jordan, G.L. Surgical Diseases of the Pancreas; Pitman Medical Publishing: London, UK, 1960.
- Sommerville, C.A.; Limongelli, P.; Pai, M.; Ahmad, R.; Stamp, G.; Habib, N.A.; Williamson, R.C.; Jiao, L.R. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: An analysis of clinicopathological factors. J. Surg. Oncol. 2009, 100, 651–656.
- Yeo, C.J.; Cameron, J.L.; Sohn, T.A.; Lillemoe, K.D.; Pitt, H.A.; Talamini, M.A.; Hruban, R.H.; Ord, S.E.; Sauter, P.K.; Coleman, J.; et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: Pathology, complications, and outcomes. Ann. Surg. 1997, 226, 248–257.
- Bouvet, M.; Gamagami, R.A.; Gilpin, E.A.; Romeo, O.; Sasson, A.; Easter, D.W.; Moossa, A.R. Factors influencing survival after resection for periampullary neoplasms. Am. J. Surg. 2000, 180, 13–17.
- Ahmad, S.R.; Adler, D.G. Cancer of the ampulla of vater: Current evaluation and therapy. Hosp. Pract. 2014, 42, 45–61.
- Ahn, D.H.; Bekaii-Saab, T. Ampullary cancer: An overview. Am. Soc. Clin. Oncol. Educ. Book 2014.
- Kobayashi, A.; Konishi, M.; Nakagohri, T.; Takahashi, S.; Kinoshita, T. Therapeutic approach to tumors of the ampulla of Vater. Am. J. Surg. 2006, 192, 161–164.
- O’Connell, J.B.; Maggard, M.A.; Manunga, J., Jr.; Tomlinson, J.S.; Reber, H.A.; Ko, C.Y.; Hines, O.J. Survival after resection of ampullary carcinoma: A national population-based study. Ann. Surg. Oncol. 2008, 15, 1820–1827.
- Kim, A.L.; Choi, Y.I. Safety of duodenal ampullectomy for benign periampullary tumors. Ann. Hepato-Biliary-Pancreat. Surg. 2017, 21, 146–150.
- Jung, Y.K.; Paik, S.S.; Choi, D.; Lee, K.G. Transduodenal ampullectomy for ampullary tumor. Asian J. Surg. 2021.
- Park, J.S.; Yoon, D.S.; Park, Y.N.; Lee, W.J.; Chi, H.S.; Kim, B.R. Transduodenal local resection for low-risk group ampulla of vater carcinoma. J. Laparoendosc Adv. Surg. Tech. A 2007, 17, 737–742.
- Dittrick, G.W.; Mallat, D.B.; Lamont, J.P. Management of ampullary lesions. Curr. Treat. Options Gastroenterol. 2006, 9, 371–376.
- Sauvanet, A.; Dokmak, S.; Cros, J.; Cazals-Hatem, D.; Ponsot, P.; Palazzo, M. Surgical Ampullectomy with Complete Resection of the Common Bile Duct: A New Procedure for Radical Resection of Non-invasive Ampulloma with Biliary Extension. J. Gastrointest. Surg. 2017, 21, 1533–1539.
- Song, J.; Liu, H.; Li, Z.; Yang, C.; Sun, Y.; Wang, C. Long-term prognosis of surgical treatment for early ampullary cancers and implications for local ampullectomy. BMC Surg. 2015, 15, 32.
- Winter, J.M.; Cameron, J.L.; Olino, K.; Herman, J.M.; de Jong, M.C.; Hruban, R.H.; Wolfgang, C.L.; Eckhauser, F.; Edil, B.H.; Choti, M.A.; et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: Implications for surgical strategy and long-term prognosis. J. Gastrointest. Surg. 2010, 14, 379–387.
- Gao, Y.; Zhu, Y.; Huang, X.; Wang, H.; Huang, X.; Yuan, Z. Transduodenal ampullectomy provides a less invasive technique to cure early ampullary cancer. BMC Surg. 2016, 16, 36.
- Lee, H.; Park, J.Y.; Kwon, W.; Heo, J.S.; Choi, D.W.; Choi, S.H. Transduodenal Ampullectomy for the Treatment of Early-Stage Ampulla of Vater Cancer. World J. Surg. 2016, 40, 967–973.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
679
Revisions:
3 times
(View History)
Update Date:
28 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




