1. Sol-Gel Methods
The sol-gel approaches
[1] are among the most investigated techniques applied to obtaining ceramic or glass materials, having the advantages of being reproducible, industrially scalable and highly controllable.
The soft template processes underlying sol-gel strategies are generally based on several steps: (i) preparation of the solution of a selected TiO2 precursor; (ii) hydrolysis of TiO2 precursor in the presence of a suitable surfactant; (iii) removal of the solvent in order to facilitate the generation of the gel; (iv) condensation reaction; and (v) calcination for the complete removal of surfactant, solvent and unreacted precursor.
Among the sol-gel synthetic approaches the EISA (evaporation-induced self-assembly, ) has been recently applied for the preparation of metal oxides including TiO
2. The main feature of the EISA method is the use of a surfactant as a templating agent. Triblock copolymers as P123 (Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)) and F127 (poly(ethylene oxide) poly(propylene oxide)-poly(ethylene oxide)), are recognized as the most promising surfactants used for this method
[2]. Indeed, surfactant selection represents one of the most critical parameters of EISA approaches because its chemical and physical properties affect the textural properties of the resulting material that can be deposited as a thin film on a suitable substrate.
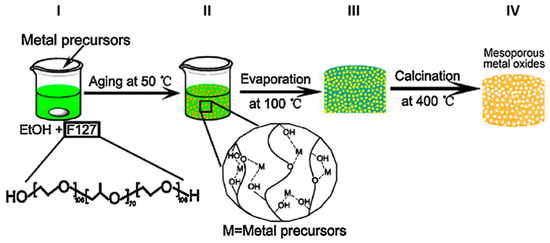
Figure 1. General synthetic scheme for the production of mesoporous metal oxides according to the evaporation-induced self-assembly method (EISA). The first step consists in the preparation of an ethanol solution containing the metal precursor (Ti(OBu)
4 for TiO
2) and the Pluronic F127 as templating agent (
I). The mixture is kept at 50 °C for 24 h in order to induce the coordination bonds between the metal ions (M) and oxygen-containing group of F 127 (
II). The subsequent thermal treatment at 100 °C for 6 h (
III) promotes the formation of a xerogel of the metal-F127 hybrids. The final calcination at 400 °C (
IV) is intended to remove of organic molecule and results in the formation of mesoporous metal oxides. Reprinted with the permission of ref.
[3]. Copyright © 2019 4542370045937.
A typical sol-gel EISA synthesis of TiO
2 starts with the preparation of a solution containing Pluronic F127 in absolute alcohol (EtOH), and the subsequent addition of titanium butoxide Ti(OBu)
4 under vigorous stirring (, I). The resulting suspension is kept at 50 °C for 24 h, and then dried at 100 °C for 6 h (, II and III respectively). The as-prepared product shows a texture compatible with xerogels. The final calcination at 400 °C is carried out at specific heating rate in order to induce the removal the block copolymer surfactant species (, IV). At this stage, aggregates formed by NPs of 5–10 nm in size have been produced, thus resulting in a mesoporous product with a specific surface area of 145.59 m
2/g and an average pore size of 9.16 nm
[3].
M.G. Antoniou et al. reported a similar approach to obtain a mesoporous TiO
2-based coating for photocatalytic applications. The TiO
2 sol, comprised of titanium tetraisopropoxide (TTIP), acetic acid, isopropanol and Tween 80 as surfactant, is applied by dip-coating on glass substrate and then it is heated at 500 °C to remove the surfactant template. The dip-coating–calcination cycle is repeated 3 times for each deposition, resulting in uniform and transparent mesoporous nanocrystalline TiO
2 films with high surface area (147 m
2/g), porosity (46%) and anatase crystallite size of 9.2 nm. The amount of photocatalyst per cm
2 is estimated to be 62.2 mg/cm
2 with an overall coated area, considering both sides of the substrate, of 22.5 cm
2 [4].
An alternative strategy has been proposed to further increase the specific surface area of mesoporous TiO
2, that indicates the use of two types of TiO
2 precursors such as TiCl
4 and TTIP in a suitable molar ratio, with TiCl
4 playing the two-fold role of precursor and pH stabilizer. A solution containing a defined TiCl
4:TTIP:P123:ethanol ratio is stirred for 3 h at room temperature and the resulting product is suitable to be deposited by spin coating on glass substrates. After drying at room temperature for 24 h, the samples is thermally treated at 130 °C for 2 h to promote crossing-linking and prevent possible cracks in the film and collapsing of the mesostructure due to the high temperature. The final calcination treatment is carried out by heating stepwise up to 400 °C
[5].
A recently reported sol-gel synthetic approach for the production of TiO
2 makes use of a biological template, namely the bacteriophage M13, a rod-shaped virus that is able to control the alkoxide condensation in the sol-gel process allowing the formation of mesopores having a diameter that can be tuned by adjusting only the reaction pH. Remarkably, the resulting product exhibits exceptional thermal stability of the anatase phase, which stays as the predominant phase even after a thermal treatment at 800 °C, that, in fact, promotes an increase in the pore and crystal size ()
[6].
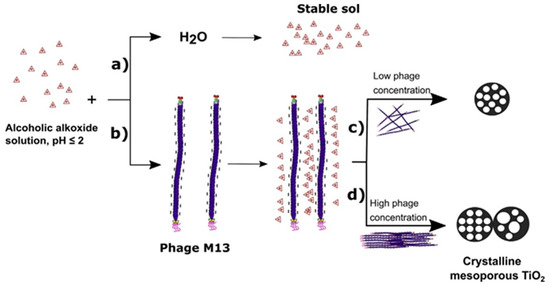
Figure 2. Proposed mechanism of mesoporous TiO
2 synthesis: consist in the preparation of a titanum alkoxide (titanium(tetra)isopropoxide) solution at pH ≤ 2. A vary stable sol is obtained with acid aqueous solution (pH 1–2) (
a); the sol-gel reaction is performed with Phage M13 and a well-established structure is obtained (
b). A local order of pores and macropores can be obtained at high phage concentration (
d), while disordered pores with a narrow pore size distribution at a low concentration (
c). Reproduced with permission from
[6]. Copyright © 2019 4541961016973.
One of the main goals in the synthesis of mesoporous TiO
2 for environmental photocatalytic applications is to increase the TiO
2 optical response in the range of visible light. For this purpose, synthetic approaches have been developed to accomplish this result. For instance, a mixture of polyethylene glycol (PEG) and polyacrylamide (PAM), has been used as the templating agent. PAM and PEG are slowly introduced in a mixture of deionized water, nitric acid (8%), ethanol and Ti(OBu)
4 as TiO
2 precursor. The resulting white gel is dried until a light-yellow powder is obtained that undergoes two calcination steps: the first in nitrogen atmosphere, and the second in air. In the first calcination step, three different temperature values are investigated: 500 °C, 600 °C and 700 °C respectively, while the second calcination step is carried out at 500 °C. The authors have demonstrated how increasing PAM mass the gel formation rate increases, due to the improved interaction between amide groups of PAM with the hydroxyl groups of the TiO
2 sol. The PEG prevents the mesostructure collapsing during the first thermal treatment. Moreover, the molecular weight (MW) of PEG has been reported to increase as the crystallite size increases and the specific surface area decreases. The obtained mesoporous TiO
2 is found to have a specific surface area measured by BET (Brunauer–Emmett–Teller) test between 104.25 and 110.73 m
2/g and a pore size (measured by Barret-Joyner-Halenda isotherm) between 16.92–16.80 nm; being the variation of the specific surface area and pore size values affected by the variation of the molecular weight of the PEG used in the synthesis. The authors point out that the two calcination steps improve the textural properties of the TiO
2 because they promote a higher crystallinity, and allow to achieve a homogenous porosity, and a higher specific surface area. In particular, the first calcination step under N
2 atmosphere causes the conversion of PEG (less thermally stable than PAM) in amorphous carbon, which plays the role of a scaffold around pores, thus preventing the mesostructure from collapsing
[7]. Furthermore, the small amount of amorphous carbon is able to induce a doping effect, and therefore the obtained photocatalyst is able to extend its photoactivity to visible range, as demonstrated in the ultraviolet–visible (UV–Vis) reflectance spectrum, that shows an increase, in the visible range, of the Kubelka–Much function intensity.
Also, Phattepur et al. have synthesized mesoporous TiO
2 with an innovative sol-gel technique by using lauryl lactyl lactate as biodegradable and inexpensive additive to control the size of large inorganic cluster. The nanostructured photocatalyst is prepared by using Ti(OBu)
4 as precursor in a solution containing a defined amount of lauryl lactyl lactate (0.25 mL, 0.5 mL, 0.75 mL and 1 mL), ethanol, and hydro-chloric acid. Such a solution is mixed with a second solution of ethanol and distilled water under vigorous stirring for 8 h up to the generation of the gel. The mixture is, then, aged, dried, ground and finally calcined, resulting in a final product with a specific surface area up to 40.10 m
2/g, a pore volume 0.112 cm
3/g
[8].
The interest towards colloidal routes for the synthesis of mesoporous TiO
2 is further supported by a recently granted US patent
[9]. The synthetic scheme consists of an acid-catalyzed hydrolysis of a water soluble TiO
2 precursor as TiOCl
2 or TiOSO
4, occurring in the presence of a porogen molecule, namely an organic alpha hydroxyl carboxylic acid, as citric acid, at relatively low temperatures (up to 100 °C). Such a procedure allows 100 nm spherical mesoporous anatase NPs to be achieved and control of the pore size by varying the molar ratio between the TiO
2 precursor and the organic alpha hydroxyl acid. Remarkably, these mesoporous TiO
2 NPs show a bimodal pore size distribution. Such bimodal porosity is due to the presence of both intra-particle and inter-particle pores. Intra-particle pores (from 2 nm to 12 nm in size) are detected in individual TiO
2 NPs while the inter-spatial fissures of dimensions from 15 nm to 80 nm, due to the packed arrangement of the NPs, result in inter-particle pores
[9].
3. Hydrothermal Synthetic Methods
A typical hydrothermal process is carried out in an autoclave, possibly equipped with Teflon liners and a closed system under controlled temperature and/or pressure (room temperature and at pressure >1 atm)
[17]. The temperature can be higher than the boiling point of water corresponding to the pressure of vapor saturation
[18].
Hydrothermal processes are extremely attractive for the large-scale production of mesoporous TiO
2 NPs, because they (i) are environmentally friendly, (ii) make use of aqueous solutions, (iii) do not require any post-calcination treatment and (iv) allow a facile recovery of the photocatalyst after the synthesis
[18][19][20].
TiO
2 NPs prepared by hydrothermal methods show several advantages, including high crystallinity, reduced particle size, uniform size distribution, prompt dispersibility in polar and non-polar solvents and a stronger interfacial adsorption; moreover, they enable the easy fabrication of high-quality coatings on several supporting material
[21].
In hydrothermal synthesis, temperature, filling volume
[22] pressure
[23] pH and treatment duration are regarded as the key parameters to control the resulting morphological and structural properties of TiO
2. Under hydrothermal conditions, a decrease of the reaction temperature cause a particle size decrease and an increase of particle agglomeration
[17]. The growth of TiO
2 NPs is also possible by using a template-based technique, taking advantage of the use of suitable high-molecular-weight surfactants, able to promote structural self-assembly
[17].
The hydrothermal method applied on TiO
2–nH
2O amorphous gel, considered as TiO
2 NPs precursor, has been widely used to prepare nanocrystalline titania
[24]. This treatment can be carried out either in pure distilled water or in the presence of mineralizing species, such as hydroxides, chlorides and fluorides of alkali metals at different pH values
[24]. Kolen’ko et al. have developed the synthesis of ultrafine mesoporous titania powders in anatase phase via hydrothermal process starting from TiO
2–nH
2O amorphous gel. The preparation of TiO
2–nH
2O amorphous gel requires multiple steps starting from the high-temperature hydrolysis of complex titanyl oxalate acid (H
2TiO(C
2O
4)
2) aqueous solutions. Briefly, the preparation route of H
2TiO(C
2O
4)
2 aqueous solution is based on: (I) the preparation of H
2TiCl
6 from TiCl
4 and chloride acid (HCl), (II) hydrolysis of H
2TiCl
6, (III) wash of TiO
2–nH
2O by distilled water, and (IV) dissolution of TiO
2–nH
2O in the oxalic acid. At this stage the TiO
2–nH
2O is treated in a polytetrafluoroethylene (PTFE)-lined autoclave at temperature of 150 °C or 250 °C for a period of time ranging from 10 min to 6 h. After the treatment in autoclave the sample is cooled down to room temperature, the obtained product is centrifuged, washed and dried at 80 °C
[24]. Washing procedures are essential in order to collect the photocatalyst and remove the templating agent and salts that can possibly obstruct the pores, thus achieving the desired porosity and specific surface area
[25][26]. The size of the mesoporous anatase particles can be controlled in the range of 60–100 nm by adjusting the concentration of H
2TiO(C
2O
4)
2 aqueous solution that is expected to affect the amount of TiO
2 nuclei.
Hydrothermal synthesis allows also a fine control over NP morphology. Indeed, mesoporous TiO
2 microparticles with a well-defined spherical shape in the range of 2–3 µm, and a crystallite size in the range from 7.3 nm to 22.3 nm have been prepared by hydrothermal reaction of poly(ethylene glycol)-poly(propylene glycol)-based triblock copolymer and TTIP mixed with 2,4-pentanedione
[27]. The surfactant solution is prepared dissolving the triblock copolymer in distilled water at 40 °C and adding sulfuric acid. Successively, TTIP is mixed with 2,4-pentanedione and slowly added dropwise into the previously prepared surfactant solution. The reaction is carried out at 55 °C for 2 h without stirring and a light-yellow powder is obtained. The hydrothermal treatment is performed at 90 °C for 10 h followed by an annealing step, which is necessary to remove the residual surfactant.
Zhou and co-workers have proposed titanium sulfate (Ti(SO
4)
2) as a precursor of TiO
2 in the presence of urea to obtain microspheres by hydrothermal treatment TiO
2 [28], by using reaction time as a key parameter to control average crystallite size, pore size and volume, and the specific surface area.
Mesoporous TiO
2 anatase microspheres have been also successfully obtained with a simple one-step hydrothermal synthesis by Lee et al.
[19]. The titanium-peroxo complex is treated with nitric acid, 2-propanol, NH
4OH in Teflon-lined autoclave at 120 °C for 6 h. The final product is filtered, washed with distilled water several times until the pH reaches 7 and dried at 65 °C for 1 day, obtaining the mesoporous material without any post-calcination procedure. In particular, the size of mesoporous TiO
2 anatase microspheres lays in a dimensional range between 0.5 µm and 1 µm. According to high-resolution TEM (HRTEM) analysis, the formation of a microsphere (secondary particle) results from the aggregation of diamond-shaped TiO
2 NCs (50 nm × 20 nm) (primary NPs). The authors have suggested that during the hydrothermal reaction the aggregation of individual diamond-shaped TiO
2 NCs in secondary particles, is thermodynamically favored with respect to the formation of primary TiO
2 NPs. Interestingly, the porosity of the microspheres is ascribable to the interspaces between TiO
2 NPs assembled to form a microsphere
[19].
Similarly, Santhosh et al. have synthesized mesoporous TiO
2 microspheres by a facile hydrothermal reaction carried out at 120 °C for 24 h that, instead, makes use Ti(OBu)
4 as precursor
[29]. The material structure is based on TiO
2 having a diameter in a range from 100 nm to 300 nm. The corresponding specific surface area is 56.32 m
2/g and the bimodal pore structure shows pore width of 7.1 nm and 9.3 nm respectively
[29].
Hollow TiO
2-based core-shell structures have been also reported and they are expected to show a high photocatalytic activity because their unique morphology allows the multiple reflection of UV light within the inner cavity
[30]. Furthermore, they display as valuable advantages high surface-to-volume ratio, low density and low production cost. Recently, Cui et al. have reported a facile one-step hydrothermal method to synthetize a mesoporous hollow core-shell structured TiO
2 microspheres. PEG (MW 2000) has been used as soft templating agent to obtain Ti
4+-PEG globules suited for the generation of hollow structures. The control on crystallite size, microsphere size, shell thickness and the roughness are achieved by tuning the duration of the hydrothermal treatment. In particular, increasing the reaction time, the crystallite and microsphere size, the shell thickness and the roughness increase while the core size decreases
[30].
Ye et al. have developed an interesting strategy for the large–scale synthesis of hallow microspheres by performing a hydrothermal treatment followed by a calcination step ()
[31]. They have used potassium titanium oxalate (PTO) as Ti precursor performing the hydrothermal process in autoclave at 150 °C for 4 h, thus obtaining 1.75 µm microspheres composed of TiO
2 NPs. In particular, a strong effect of calcination temperature on the photocatalytic performance of the photocatalyst is reported, with the hollow TiO
2 microspheres calcined at 500 °C showing the highest photocatalytic activity.
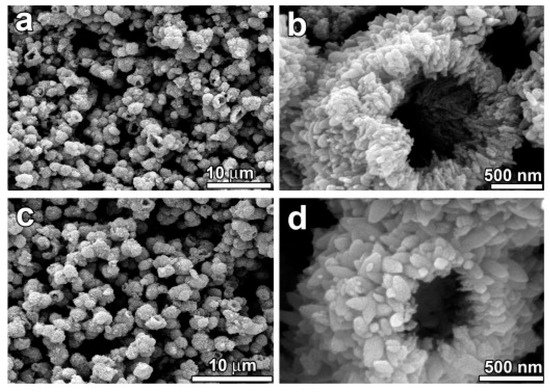
Figure 3. Large–scale synthesis of TiO
2 hallow microspheres has been obtained by Ye et al. by using a hydrothermal treatment following the calcination. The morphology and microstructure of the hollow microspheres have been characterized by scanning electron microscopy (SEM) (
a–
d), in order to evaluate the effect of the calcination. (
a,
b) SEM micrographs of the not calcined sample: low-magnification and high-magnification, respectively. (
c,
d) SEM micrographs of the TiO
2 hollow microspheres calcined at 500 °C for 2 h: low-magnification and high-magnification, respectively. SEM micrographs of the non-calcined material show that the sample consisting of large-scale hollow microspheres, being the shell of the microsphere itself made of numerous nanoparticles (NPs). Interestingly, the SEM micrographs of calcined material indicate an excellent thermal stability of the hallow microsphere. The morphology and the mean external diameter of the hollow microspheres has been found unchanged after the calcination treatment at 500 °C. Reproduced with permission from
[31]. Copyright © 2018 Elsevier.
TiO
2 hollow spheres aggregates, characterized by porous walls, can be also synthesized on large-scale by performing the hydrothermal hydrolysis of Ti(SO
4)
2 assisted by NH
4F without employing any templates
[32]. The resulting hollow material, reported in , presents a high surface area, smaller crystal size, and highly porous structure
[32] Several hydrothermal protocols have been also successfully developed to synthesize anisotropic mesoporous NPs. Anisotropic TiO
2 NCs have been extensively investigated for several energy conversion applications, since their peculiar morphology enables a facile charge transport along the longitudinal dimension and decreases the e
−/h
+ recombination rate
[21][22].
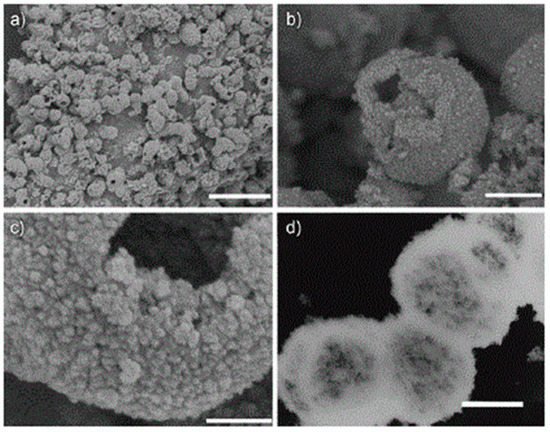
Figure 4. Large-scale synthesis of porous TiO
2 hollow aggregates reported by Liu et al. The synthesis is carried out using a low-temperature hydrothermal method without templates. (
a–
c) Field-emission scanning electron microscopy (FESEM) analyses at different magnifications and (
d) transmission electron microscope (TEM) micrograph of the “as prepared” sample by one-step hydrothermal treatment at 160 °C for 6 h. The scale bars for (
a–
d) are 5 mm, 500 nm, 100 nm, and 500 nm, respectively. The low-magnification FESEM micrograph (
a) of the sample indicates that the aggregates are composed of a large amount of NPs. While, the micrograph (
b) shows the hollow interior of the single aggregate and the micrograph (
c) at a higher magnification highlights the porous structure of the TiO
2 NP aggregates. Moreover, the hollow structure of the TiO
2 sample is confirmed by TEM micrograph (
d). Reproduced with permission from
[32]. Copyright © 2018 John Wiley and Sons.
Mesoporous TiO
2 nanotubes (TNTs) are materials of great interest due their high ion-exchange capability, relative stability, enhanced conductivity and high specific surface area
[33][34]. In addition, TNTs contain a large amount of hydroxyl groups respect to spherical TiO
2 and may find an effective use as ion adsorbent systems. Sattarfard et al. have prepared mesoporous TNTs by hydrothermal synthesis starting from TiO
2–P25 NPs as titania precursor
[33].
In this protocol, TiO
2–P25 powder is introduced into NaOH aqueous solution and held at 115 °C for 24 h in a stainless-steel with Teflon lining reactor placed in an oil bath. Afterwards, the mixture is cooled to room temperature and centrifuged, and the obtained precipitate requires several washing, recovery and thermal treatment steps to finally obtain TNT with a specific surface area of 200.38 m
2/g and an average outer and inner diameter approximately of 9 nm, 4 nm, respectively, with a wall thickness of 2.5 nm. Remarkably, the use of a strong aqueous base as NaOH as dispersing agent for TiO
2 P25 and the subsequent hydrothermal treatment are the key steps that determine the formation of titanate tubular structures, while washing with HCl has been demonstrated to be essential to convert tubular titanate in TiO
2 nanotubes. Indeed, the treatment of TiO
2 NPs with NaOH is known to break Ti–O–Ti bonds, thus resulting in the formation of sheets, while the subsequent washing with acid or water, reducing the electrostatic charge, induces the folding of the sheets yielding the formation of nanotubes
[33].
TiO
2 nanowires can be also successfully obtained by using hydrothermal method, resulting in a fine shape control, as proposed by Asiah et al. that investigated a low-cost, high-purity shape-controlled synthetic strategy for the large-scale production of mesoporous TiO
2 nanowires
[35]. Titanium (IV) oxide nanopowder is used as Ti precursor solution and treated with NaOH aqueous solution. The treatment is performed by following a hydrothermal route in autoclave in a Teflon beaker at 150 °C for a reaction time ranging from 1 to 10 h. The obtained white precipitate is washed with HCl and deionized water until pH = 7 is reached. The final product, dried at 40 °C overnight and annealed at 500 °C, is found to consist of few microns long nanowires with diameters from 15 to 35 nm, according to the duration of the treatment (). Interestingly, the hydrothermal method has also been shown to be powerful for synthesizing a three-dimensional (3D) TiO
2 mesoporous superstructure
[36][37]. TiO
2 based superstructures with a branched architecture present an enhanced specific surface area, improved charge separation and transfer within the TiO
2 branches, thus increasing the e
−/h
+ pairs lifetime and, therefore, the ROS generation
[38].
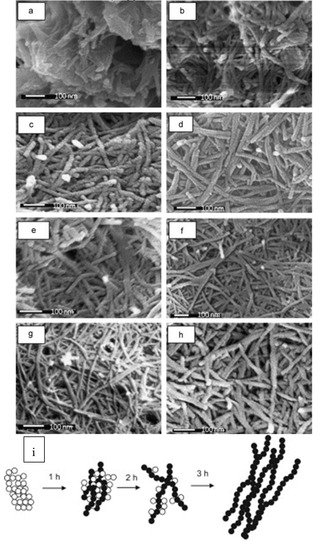
Figure 5. Large-scale hydrothermal synthesis is explored to produce surfactant-free seed mediated mesoporous TiO
2 nanowires by Asiah et al. (
a–
h) FESEM micrographs of TiO
2 nanowires at different growth time, (
a) 1 h, (
b) 2 h, (
c) 3 h, (
d) 4 h, (
e) 5 h, (
f) 6 h, (
g) 8 h and (
h) 10 h, respectively. (
i) Schematic diagrams of growth evolution of TiO
2 nanowires in time. The effect of hydrothermal growth time on the evolution of the morphology and structural properties of mesoporous TiO
2 nanowires is shown. The initial morphology of as-prepared TiO
2 reacted for 1 h (
a) consists of unreacted NPs which are agglomerated and small yield of the formed nanowires with very low aspect-ratio. After 2 h (
b) the structure of nanowires is clearly formed. However, the NPs are completely converted into nanowires after 3 h (
c). Reproduced with permission from
[35]. Copyright © 2018 Elsevier.
Baloyi et al. have proposed the hydrothermal synthesis of dandelion-like structures from TiCl
4 and water via a simple hydrothermal
[38] synthesis realized in a reaction flask by adding drop-wise TiCl
4 to the super-cooled high purity water, by using a separator funnel under vigorous stir and heating at 100 °C for 24 h. Successively, the suspension is centrifuged and washed with water to remove any chloride ions from the solid TiO
2 and the resulting solid is dried for 16 h overnight at 120 °C. It has been proposed the obtained nanostructures grow according to a four-step reaction mechanism: (i) nucleation and NP formation; (ii) formation of spheres; (iii) further growth; and (iv) formation of flower-like TiO
2 structures by agglomeration of the dandelions.
Pan et al. have reported the large–scale synthesis of uniform urchin-like mesoporous TiO
2 hollow spheres (UMTHS) by a hydrothermal method based on targeted etching of self-organized amorphous hydrous TiO
2 solid spheres (AHTSSs) ()
[37]. The growth of the UMTHSs under hydrothermal conditions has been proposed to start from the spontaneous reconstruction of surface–fluorinated AHTSSs in the presence of surface coating of polyvinylpyrrolidone (PVP). Briefly, the previously synthesized AHTSSs are treated with NaF, used as etching agent and successively with PVP. After an hour of stirring, the suspension is transferred to a Teflon-lined autoclave and kept at 110 °C for 4 h. The UMTHSs are obtained by collecting, washing with diluted NaOH solution and water, and finally calcining at 350 °C for 2 h.
Figure 6. Large-scale synthesis of urchin-like mesoporous TiO
2 hollow spheres (UMTHS) by low-temperature hydrothermal method. SEM (
a) and TEM (
b–
e) micrographs of UMTHS obtained by calcining the powder after hydrothermal reaction. SEM micrographs shows that the UMTHS are monodisperse with uniform particle size and consist of radially arranged anatase nanothorns, assembling an urchin-like shaped hierarchical structure. Furthermore, TEM analysis (
b,
c) indicates that the spherical shell consisting of radial nanothorns. The single hallow sphere is polycrystalline due to radial orientation of nanothorns, as revealed by selected-area electron diffraction (inset of
d). Moreover, the high-resolution TEM (
e) shows that each nanothorn presents a single-crystal nature and possesses the lattice fringes of anatase (101) plane with a d -spacing of 0.35 nm aligned over the single nanothorn. Reproduced with permission from
[37]. Copyright © 2018 John Wiley and Sons.
The inner structures of the materials have been demonstrated to be tunable from the conventional solid spheres to hollow and complex core-shell and yolk-shell configuration, by varying the experimental parameters and suitably protecting the inner structure by prefilling AHTSSs with PEG. The synthesized structures show a large surface area up to 128.6 m
2/g and excellent photocatalytic performances for environmental application
[37].
Recently, Hu et al. have employed one-step hydrothermal method to synthetized 3D flower-like TiO
2 microspheres using Ti(OBu)
4 as titanium source and glacial acetic acid (HAc) as solvent and capping agent, at the same time
[39]. Briefly, a solution of Ti(OBu)
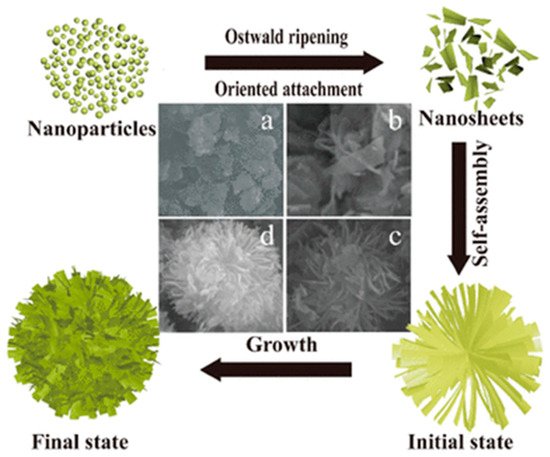
4 and HAc is prepared and, after stirring, is transferred into a Teflon-lined stainless-steel autoclave and heated at 140 °C for a defined period. The resulting product is collected upon centrifugation, washed with ethanol and deionized water repeatedly, dried at 60 °C for 12 h, and annealed in air. The 3D flower-like structures are formed due to the oriented assembly of nanosheets as reported in .
Figure 7. Schematic illustration of the growth evolution mechanism for 3D flower-like TiO
2 microspheres self-assembled by nanoplates using the hydrothermal method at different time: (
a) 3 h; (
b) 6 h; (
c) 9 h; (
d) 12 h. TiO
2 NPs after the nucleation begins to grow in the direction of orientation, forming nanosheets after dehydration. With the increasing of hydrothermal time (at 400 °C), the nanosheets are self-assembled to form a 3D flower-like structure as final state. Reproduced with permission from
[39]. Copyright © 2018 Springer Nature.
TiO
2 based photocatalyst with wormhole-like disordered structure was hydrothermally prepared by using titanium sulfate (Ti(SO
4)
2) as precursor of TiO
2, and cetyltrimethyl ammonium bromide (CTAB) as a structure-directing agent
[40]. The resulting photocatalyst is characterized by a narrow pore size distribution and a very high surface area of 161.2 m
2/g, presenting an excellent adsorption ability for the removal of methyl orange (MO) and Cr(VI) from wastewater. The Ti(SO
4)
2 has been used as TiO
2 precursor also for the hydrothermal synthesis of hollow TiO
2 microspheres composed of 60 nm sized nanospheres thus resulting in a large hierarchical nanostructure characterized by a surface area and pore volume of 123 m
2/g and 0.19 cm
3/g, respectively
[41].