
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sivarama Krishna Lakkaboyana | + 1244 word(s) | 1244 | 2021-05-24 05:15:49 | | | |
| 2 | Lindsay Dong | Meta information modification | 1244 | 2021-07-22 03:26:55 | | |
Video Upload Options
Industrial wastewater originating from various industries contributes as a major source of water pollution. This pollutant poses a severe threat to the environment. Recent years saw nanomaterials as a potential candidate for pollutants removal. Nowadays, a range of cost-effective nanomaterials is available with unique properties.
1. Introduction
Figure 1 and Figure 2 shows wastewater and industrial wastewater general classifications. When left untreated, these constituents may pose a threat to living beings and the environment, which makes it crucial to treat wastewater before disposal. Various physical, chemical, and biological treatment processes are used for wastewater treatment. Until now, a variety of research on nanomaterials was done to research heavy metal water treatments to find their applications, and they show incredible potential as an irreplaceable option to adsorb heavy metals from wastewater [1]. For the removal of heavy metals from polluted wastewater, these properties are very useful. According to the type of nanomaterial, wastewater treatment is classified into three fundamental groups [2]: nano-adsorbents, nanomembranes, and nano-catalysts. Figure 3 shows the steps in wastewater treatment processes as a flow chart.
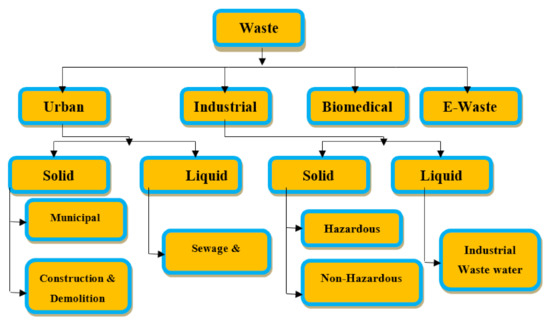
Figure 1. Anthropogenic wastes classification, (Reprinted with permission from [1]).
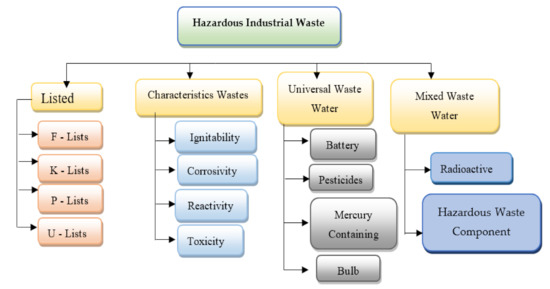
Figure 2. Industrial hazardous wastes general classification (reprinted with permission from [1]). F-list (wastes from common manufacturing and industrial processes), K-list (wastes from specific industries), P-list, and U-list (wastes from commercial chemical products).
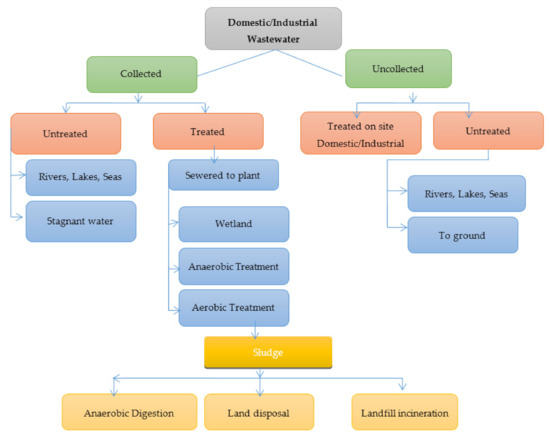
Figure 3. Flow chart showing steps in wastewater treatment processes.
2. Removal of Dyes
From industries, waters dyes and pesticides are being released frequently, and their findings, particularly at low concentrations, need the development of complex advances, for example, separation or filtration of compound combinations combined with detection utilizing multi-technique methods [3][4]. These estimations are subsequently tedious due to the many middle-level handling steps associated with preparing the sample. Utilization of silicon-graphene (sg) nanoporous composites takes into consideration an extreme cut of all these methods, as the compounds can pre-concentrate the analyte into the porous structure and widen the analyte signal if a proper method is utilized [5]. Table 2 shows the application and the harmfulness of various dyes [6][7].
| Dyes | Example | Advantages | Toxicity |
|---|---|---|---|
| Acid | Methyl orange, Sunset yellow | Wool, paper, leather, silk | Carcinogenic |
| Cationic | Rhodamine 6g, Methylene blue | Paper modified polyesters | Carcinogenic |
| Direct | Congo red, Direct red 23 | Cotton, paper, leather | Bladder cancer |
| Disperse | Disperse red, Disperse orange 3 | Nylon, acrylic fibers | Skin allergenic |
| Reactive | Reactive red 198 Reactive red 120 |
Nylon, wool, cotton | Dermatitis, allergic conjunctivitis |
| Vat | Vat orange 28, Vat orange 50 | Cellulosic fibers |
The adsorption was helped because of the substance co-operations between the composite and the functional groups; moreover, the strong π bonding between composites and the phenyl ring preferred the adsorption. Amino group surface functionalization of the multifunctional silicon graphene nanocomposites might help increase the interactions for the pollutant removal.
3. Heavy Metals Removal
Nanomaterials of metal compounds displayed preference for heavy metals removal over activated carbon, e.g., titanium dioxide nanoparticles in arsenic adsorption and nanosized magnetite. The photo catalyst usage of, for example, nanoparticles titanium dioxide, was explored in detail to decrease the toxic metal ions in water. In a survey, titanium dioxide nanocrystalline showed adequacy in eliminating various types of As, and it was demonstrated to be the most viable photo catalyst aside from industrially accessible nanoparticles of titanium dioxide, which showed almost extreme efficiency of arsenic removal at a relatively neutral pH value [8]. A titanium dioxide nanocomposite and nanoparticles of titanium dioxide added on a graphene sheet was additionally utilized to decrease chromium VI to chromium III in daylight. Comparatively, chromium treatment was completed by utilizing nanoparticles of palladium in another survey; conventional technologies for heavy metal removal are shown in Figure 4. The removing capacity of arsenic (heavy metal) was likewise tested by utilizing Fe2O3 and Fe3O4 as adsorbents by most of the analysts. Removal of As was also additionally explored by utilizing a high, particular surface area of iron oxide nanocrystals [9]. . Figure 5, illustrates some conventional methods for removal of metal. It is a three-step treatment process.
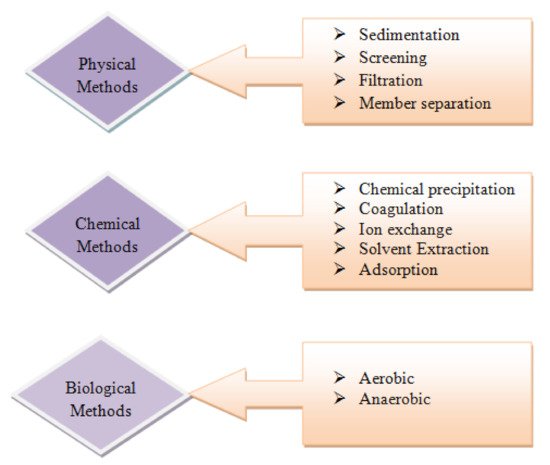
Figure 4. Conventional technologies for heavy metal removal, reprinted with permission from [10].
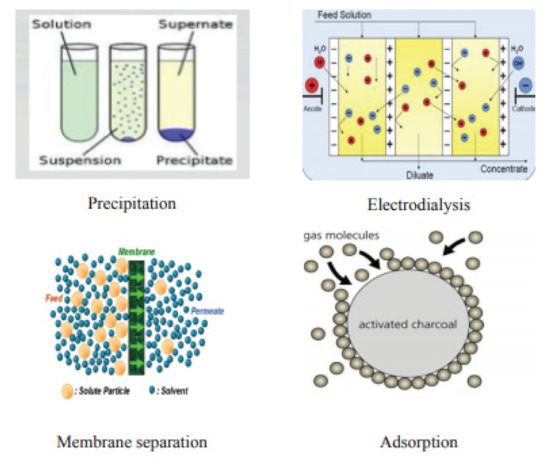
Figure 5. Conventional methods for removal of metal, reprinted with permission from [10].
4. Removal of Pesticides
Low-cost adsorbents development for pesticide maintenance is a significant part of environmental sciences research. Wastes from industries such as carbon slurry, fly ash, and sludge are delegated as easy materials due to their minimal price, and local accessibility pesticides removal can be utilized as adsorbents. Fly ash, lignite, coal-fired, thermal, power station solid waste, is an easy adsorbent that demonstrated huge adsorption limit with regard to organic contaminations [11]. Some researchers explained the sorption of pesticides and fly ash capability and suggested its utilization for pesticides removal from wastewater samples and water. Coal fly ash altogether has a high maintenance limit with regard to metolachlor, atrazine, and metribuzin.
5. Key Parameters for the Pollutant Removal
Silicon graphene multifunctional composites can be surface-functionalized to enhance the modifications with the analyte. The technique of sub-atomic is a wiser approach to silicon graphene composites with improved performance [12][13]. The molecular cavities focus on molecules and improve the detecting capabilities of dyes. The molecular imprinting is likewise useful for designing nanocomposites of silicon graphene for removing heavy metals. Moreover, conditions of the environment play a critical function in the pollutant’s adsorption.
In general, the process of sorption relies on the physical state and the physicochemical properties of the composite and the pollutant chemical composition in the water. The properties evaluation versus the performance of the material is significant due to the absence of broad availability of characterization data and associated sorption capacities, such as specific surface areas [14][15]. Regardless of these obstructions, we distinguished the patterns and were effective in building up connections that helped increase the performance of the composites.
6. Nanomaterials Reuse and Retaining
Nano-adsorbent reuse and materials recovery from an aqueous solution are profoundly hard and may cause problems to the environment; as the compounds are adsorbed, pre-treatment samples must be created, and the process of separation technologies must ensue [16]. As another option, ballistic electrons discharged from the nanostructured adsorbent material and the nonporous carbonaceous utilizing microwave irradiation may likewise annihilate the adsorbed compound. Nanomaterial holding and reutilization by membrane filtration are enabled device designs of nanotechnology, key features that allow persistent chemical usage because of the expense and general health concerns. Besides, membranes of ceramics are beneficial compared to polymeric membranes in photo catalytic applications, as they exceptionally oppose chemical oxidants and ultraviolet.
7. Environmental Significance and Future Application
As of now, there is no doubt of the nanomaterials usage efficiency in industrial wastewater treatment, however, nanomaterials impose a huge number of genuine cons because, during treatment processes, they may discharge into the surrounding environment, and they can withstand serious risks for a long time.
The market requirement is to restrict the costs of the procedure concerning the environmentally friendly nature of the referenced nanomaterials. Critical accentuation for this utilization should be given to green technology by material by-products of agricultural wastes. Numerous works in this area are needed in order to improve. The main concentration of the existing research works is that their utilization is yet to occur in the research [17][18]. Only a restricted amount of studies for brief market analyses and economic aspects is accessible. The principle market objective for the future is to improve the process of treatment on an industrial scale, which requires a significant monetary and technological method. In such a way, colleges and research labs can assume an effective part through a more official way to deal with the transfer of technology and copyright protection [19][20][21].
References
- Hanafiah, M.M.; Hashim, N.A.; Ahmed, S.; Ashraf, M.A. Removal of chromium from aqueous solutions using a palm kernel shell adsorbent. Desalin. Water Treat. 2018, 118, 172–180.
- Saleh, T.A.; Gupta, V.K. Processing methods, characteristics and adsorption behavior of tire derived carbons: A review. Adv. Colloid Interface Sci. 2014, 211, 93–101.
- Nizam, N.U.M.; Hanafiah, M.M.; Mahmoudi, E.; Halim, A.A.; Mohammad, A.W. The removal of anionic and cationic dyes from an aqueous solution using biomass-based activated carbon. Sci. Rep. 2021, 11, 8623.
- Paul, J. Nanomaterials for the Decontamination of Waste Water Containing Pharmaceutical Drugs. 2015. Available online: (accessed on 17 March 2021).
- Omole, M.A.; K’Owino, I.; Sadik, O.A. Nanostructured Materials for Improving Water Quality: Potentials and Risks. In Nanotechnology Applications for Clean Water; William Andrew: Boston, MA, USA, 2009; pp. 233–247.
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445.
- Liu, J.; Chen, H.; Shi, X.; Nawar, S.; Werner, J.G.; Huang, G.; Ye, M.; Weitz, D.A.; Solovev, A.A.; Mei, Y. Hydrogel microcapsules with photocatalytic nanoparticles for removal of organic pollutants. Environ. Sci. Nano 2020, 7, 656–664.
- Pearson, R.G. Hard and soft acids and bases—the evolution of a chemical concept. Co-ord. Chem. Rev. 1990, 100, 403–425.
- Al-Bastaki, N.M. Performance of advanced methods for treatment of wastewater: UV/TiO2, RO and UF. Chem. Eng. Process. Process. Intensif. 2004, 43, 935–940.
- Thanh, N.; Puntes, V.; Tung, L.; Fernig, D. Fifth international conference on fine particle magnetism. In Journal of Physics: Conference Series; IOP Publishing: Jakarta, Indonesia, 2005; pp. 70–76.
- Mumma, R.O.; Raupach, D.C.; Waldman, J.P.; Tong, S.S.C.; Jacobs, M.L.; Babish, J.G.; Hotchkiss, J.H.; Wszolek, P.C.; Gutenman, W.H.; Bache, C.A.; et al. National survey of elements and other constituents in municipal sewage sludges. Arch. Environ. Contam. Toxicol. 1984, 13, 75–83.
- Sakaki, K.; Sugahara, H.; Kume, T.; Ohashi, M.; Naganawa, H.; Shimojo, K. Method for Synthesizing rare Earth Metal Extractant. U.S. Patent 8841482, 23 September 2014.
- Ms, A.-R.; Arm, A.-R. Removal of Heavy Metals from Industrial Waste Water by Biomass-Based Materials: A Review. J. Pollut. Eff. Control. 2016, 5, 180.
- Ogata, T.; Narita, H.; Tanaka, M. Adsorption behavior of rare earth elements on silica gel modified with diglycolamic acid. Hydrometallurgy 2015, 152, 178–182.
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005.
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605.
- Tarabara, V.V. Multifunctional Nanomaterial-Enabled Membranes for Water Treatment. In Nanotechnology Applications for Clean Water; William Andrew Publishing: New York, NY, USA, 2009; pp. 59–75.
- Shahamat, Y.D.; Khani, M.R.; Mahdizadeh, H.; Kannan, K.; Kalankesh, L.R.; Kamarehei, B.; Baneshi, M.M. Olive Mill Wastewater (Omw) Treatment by Hybrid Processes of Electrocoagulation/Catalytic Ozonation And Biodegradation. Environ. Eng. Manag. J. 2020, 19, 1401–1410.
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007.
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851.
- Barros, Ó.; Costa, L.; Costa, F.; Lago, A.; Rocha, V.; Vipotnik, Z.; Silva, B.; Tavares, T. Recovery of Rare Earth Elements from Wastewater Towards a Circular Economy. Molecules 2019, 24, 1005.




