Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mina Tadrous | + 1182 word(s) | 1182 | 2021-07-13 10:51:46 | | | |
| 2 | Amina Yu | Meta information modification | 1182 | 2021-07-21 05:06:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tadrous, M. Direct Acting Antivirals during COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/12240 (accessed on 07 February 2026).
Tadrous M. Direct Acting Antivirals during COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/12240. Accessed February 07, 2026.
Tadrous, Mina. "Direct Acting Antivirals during COVID-19" Encyclopedia, https://encyclopedia.pub/entry/12240 (accessed February 07, 2026).
Tadrous, M. (2021, July 20). Direct Acting Antivirals during COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/12240
Tadrous, Mina. "Direct Acting Antivirals during COVID-19." Encyclopedia. Web. 20 July, 2021.
Copy Citation
We sought to quantify changes in Direct Acting Antiviral (DAA) utilization among different countries during the pandemic. We conducted a cross-sectional time series analysis between 1 September 2018 and 31 August 2020, using the IQVIA MIDAS database, which contains DAA purchase data for 54 countries.
antivirals
hepatitis C
COVID-19
drug policy
time series
1. Introduction
Close to 71 million people are chronically infected with the hepatitis C virus (HCV) worldwide [1]. If left untreated, chronic HCV infection can cause substantial morbidity and mortality, with complications including cirrhosis, end-stage liver disease, and hepatocellular carcinoma [2][3]. Published models that assume absence of treatment suggest that cases of end stage liver disease among HCV-infected people will peak between 2030 and 2035 [4]. Fortunately, treatment options for HCV infection have improved over the past decade with the introduction of direct-acting antiviral (DAA) treatment. DAAs can achieve sustained virologic responses or cure a high (>95%) proportion of patients with favorable safety and tolerance profiles [5]. Sustained virologic response leads to substantial reductions in liver transplantation, hepatocellular carcinoma, and liver-related mortality, prompting the World Health Organization (WHO) to set targets for HCV elimination by 2030 [6][7][8]. However, this goal requires sustained robust screening programs for HCV and global access to DAAs [9].
The 2019 novel coronavirus (COVID-19) pandemic has impacted all stages of the HCV cascade of care and has resulted in reduced access to critical medical services [10]. Early in the pandemic, the World Hepatitis Alliance administered a global survey assessing the effects of the COVID-19 pandemic on viral hepatitis services [11]. Findings included decreased hepatitis testing due to pandemic-related closures or patient avoidance of testing facilities and a lack of hepatitis treatment access due to temporary government-imposed restrictions in routine healthcare [11]. Although modelling studies suggest that some countries could conceivably eliminate HCV by 2030, they did not account for the COVID-19 pandemic, which may jeopardize progress toward elimination [12]. Yet the impact of the COVID-19 pandemic on global DAA utilization is unknown. We sought to quantify changes in DAA utilization among different countries during the COVID-19 pandemic.
2. Global Utilization Trends of Direct Acting Antivirals (DAAs) during the COVID-19 Pandemic: A Time Series Analysis
Overall, 46 out of 54 (85%) jurisdictions available in our database experienced a decline in DAA utilization during the COVID-19 pandemic (Figure 1). Among the 46 jurisdictions, the utilization of DAAs decreased on average by −43% (Standard Deviation (SD = 21)) during the first 6 months of the pandemic (March to August 2020). Decreases in DAA utilization ranged from −1% in Finland to −93% in Brazil. Among the eight countries that experienced a percent increase, the range was between +24% (Mexico) to +449% (South Africa). All countries in our database with a high prevalence of HCV (Egypt, Kazakhstan, Pakistan, Romania, Taiwan) experienced a −49% (SD = 27) average decline in DAA utilization. Decreases in DAA utilization in high prevalence countries ranged from −17% in Kazakhstan to −90% in Egypt.
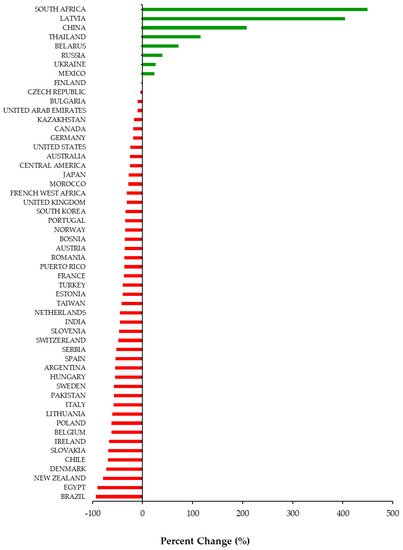
Figure 1. Percent change (%) in utilization of Direct Acting Antivirals by country and region from March to August 2020 compared to March to August 2019.
Monthly utilization of DAAs was stable across G7 countries between September 2018 to February 2020 (pre-healthcare restrictions) (Figure 2). In the early months of the pandemic (March to August 2020), all countries in the G7 experienced declines in monthly DAA utilization ranging from −58% (Italy) to −18% (Canada). However, only four countries in the G7 (Canada, Germany, the United Kingdom, and the United States of America) had statistically significant (p < 0.05) declines in utilization (Table 1, Figure 2).
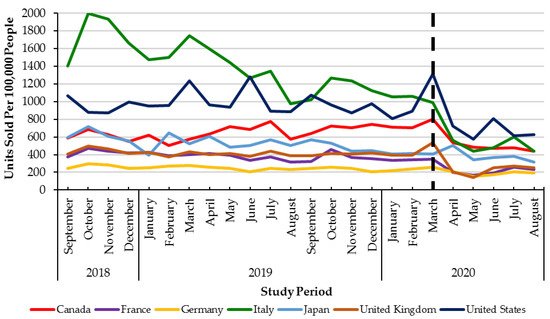
Figure 2. Direct Acting Antiviral units sold per 100,000 people from September 2018–August 2020 across the G7 countries. Dashed lined: declaration of the COVID-19 pandemic (March 2020).
Table 1. Results of interrupted time-series model examining the impact of the COVID-19 pandemic on Direct Acting. Antiviral utilization across the G7 countries.
| G7 Countries | Percent Change 1 (%) |
Change in Trend of Monthly Direct Acting Antiviral Utilization 2 |
|---|---|---|
| Canada | −18 | p = 0.0299 |
| France | −37 | p = 0.3399 |
| Germany | −19 | p = 0.0150 |
| Italy | −58 | p = 0.2008 |
| Japan | −28 | p = 0.7015 |
| United Kingdom | −32 | p = 0.0004 |
| United States of America | −24 | p = 0.0003 |
1Percent change calculation. March 2020–August 2020 compared to March 2019–August 2019. 2Time series analysis. Ramp from April 2020 to August 2020 (September 2018 to March 2020 compared to April 2020 to August 2020).
3. Discussion
We using global prescription data representing 5.4 billion people from 54 countries, shows a decline in DAA utilization associated with the COVID-19 pandemic across the majority of countries studied (46 out of 54). Importantly, all high-prevalence (HCV prevalence > 2%) countries in the database experienced declines in utilization. Across the major developed economies (the G7), we also observed a decreasing trend in DAA utilization during the early months of the pandemic that continued to remain below pre-pandemic levels by August 2020. These results highlight the impact of the pandemic to the management of HCV and may have set back the ability to achieve the WHO goal of eliminating HCV by 2030. In addition, this slowdown in HCV treatment may lead to an increased number of HCV related comorbidities during this time.
The COVID-19 pandemic has placed significant strain on national healthcare systems [13]. Although early responses to the pandemic varied across jurisdictions, the redeployment of healthcare resources and public health personnel to focus on containing the COVID-19 pandemic was common. The ensuing healthcare disruptions are known to have had far-reaching consequences for the management of patients with chronic disease, including HCV [14][15]. For example, in February 2020, the Italian government planned to conduct birth cohort screening for hepatitis; however, these programs were halted because of the early exposure to COVID-19 [16]. Similarly, in Egypt, all hepatitis screening programs were paused in March 2020 and the number of HCV treatment units operating was reduced by more than 75% [16]. Our findings build upon these early observations to provide the first estimate of a change in DAA utilization associated with the COVID-19 pandemic on a global scale.
Our results are concerning as they highlight that the pandemic may be a major speed bump on the WHO’s goal of eliminating HCV by 2030. This plan targets an 80% reduction in new chronic infections and a 65% reduction in mortality from 2015 levels [8]. Of the 45 developed countries, only 11 (Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom) were on track to meet elimination targets by 2030 [17]. A further five countries (Austria, Malta, the Netherlands, New Zealand, and South Korea) were on track to meet the targets by 2040 [17]. Interestingly, all the countries listed above that are available in our database (Iceland and Malta not in database) experienced reductions in DAA utilization. This suggests that, even prior to COVID-19, many developed countries were behind schedule to reach the elimination targets. The COVID-19 associated reductions in DAA treatment observed in our study are likely to exacerbate already delayed plans for HCV elimination. Secondly, to receive access to HCV treatment, most countries require patients to receive HCV lab testing and screening [18]. However, public health initiatives (e.g., testing and screening) have fallen behind in both developed and developing nations and need to be accounted for in future healthcare planning and re-launch strategies. This problem is of exceptional concern among the most marginalized populations in developed countries (e.g., people who inject drugs), as well as developing countries with high HCV prevalence and whereby HCV infrastructure and systems may not be as robust to make up for lost opportunities and time [19][20][21]. Consequently, restoring and ramping up HCV screening and treatment program capacity is urgently needed to mitigate the impact of COVID-19 on attaining elimination targets and optimizing the health of patients with HCV.
References
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176.
- Shepard, C.W.; Finelli, L.; Alter, M.J. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 2005, 5, 558–567.
- Shakeri, A.; Srimurugathasan, N.; Suda, K.J.; Gomes, T.; Tadrous, M. Spending on Hepatitis C Antivirals in the United States and Canada, 2014 to 2018. Value Health 2020, 23, 1137–1141.
- Buckley, G.J.; Strom, B.L. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report; The National Academies Press: Washington, DC, USA, 2016; ISBN 0309437997.
- Holmes, J.A.; Rutledge, S.M.; Chung, R.T. Direct-acting antiviral treatment for hepatitis C. Lancet 2019, 393, 1392–1394.
- Van Der Meer, A.J.; Veldt, B.J.; Feld, J.J.; Wedemeyer, H.; Dufour, J.F.; Lammert, F.; Duarte-Rojo, A.; Heathcote, E.J.; Manns, M.P.; Kuske, L.; et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. J. Am. Med. Assoc. 2012, 308, 2584–2593.
- Janjua, N.Z.; Chong, M.; Kuo, M.; Woods, R.; Wong, J.; Yoshida, E.M.; Sherman, M.; Butt, Z.A.; Samji, H.; Cook, D.; et al. Long-term effect of sustained virological response on hepatocellular carcinoma in patients with hepatitis C in Canada. J. Hepatol. 2017, 66, 504–513.
- World Health Organization (WHO). Combating Hepatitis B and C to Reach Elimination by 2030: Advocacy Brief; World Health Organization: Geneva, Switzerland, 2016; Available online: (accessed on 25 January 2021).
- Myers, S.; Khosa, G.; Kuo, I.F.; Janzen, D.; Alessi-Severini, S. Moving towards universal coverage of direct-acting antiviral therapies for hepatitis c infection in canada: An environmental scan of canadian provinces and international jurisdictions. J. Pharm. Pharm. Sci. 2018, 21, 271s–308s.
- Lanièce Delaunay, C.; Greenwald, Z.R.; Minoyan, N.; Artenie, A.A.; Jeong, D.; Marathe, G.; Saeed, Y.A.; Kolla, G.; Kunden, R.D.; Okwor, C.I.A.; et al. Striving toward hepatitis C elimination in the era of COVID-19. Can. Liver J. 2021, 4, e20200027.
- Wingrove, C.; Ferrier, L.; James, C.; Wang, S. The impact of COVID-19 on hepatitis elimination. Lancet Gastroenterol. Hepatol. 2020, 5, 792–794.
- Gamkrelidze, I.; Pawlotsky, J.M.; Lazarus, J.V.; Feld, J.J.; Zeuzem, S.; Bao, Y.; Gabriela Pires dos Santos, A.; Sanchez Gonzalez, Y.; Razavi, H. Progress towards hepatitis C virus elimination in high-income countries: An updated analysis. Liver Int. 2021, 41.
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11.
- Kondili, L.A.; Marcellusi, A.; Ryder, S.; Craxì, A. Will the COVID-19 pandemic affect HCV disease burden? Dig. Liver Dis. 2020, 52, 947–949.
- Konstantelos, N.; Shakeri, A.; McCormack, D.; Feld, J.J.; Gomes, T.; Tadrous, M. Impact of COVID-19 on prescribing trends of direct-acting antivirals for the treatment of Hepatitis, C. in Ontario, Canada. Am. J. Gastroenterol. 2021.
- Blach, S.; Kondili, L.A.; Aghemo, A.; Cai, Z.; Dugan, E.; Estes, C.; Gamkrelidze, I.; Ma, S.; Pawlotsky, J.M.; Razavi-Shearer, D.; et al. Impact of COVID-19 on global HCV elimination efforts. J. Hepatol. 2021, 74, 31–36.
- Razavi, H.; Sanchez Gonzalez, Y.; Yuen, C.; Cornberg, M. Global timing of hepatitis C virus elimination in high-income countries. Liver Int. 2020, 40, 522–529.
- Li, H.C.; Lo, S.Y. Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol. 2015, 7, 1377–1389.
- Boucher, L.M.; Bayoumi, A.M.; Mark, A.E.; Cooper, C.; Martin, A.; Marshall, Z.; Boyd, R.; Oickle, P.; Diliso, N.; Pineau, D.; et al. Hepatitis C Testing, Status and Treatment among Marginalized People Who Use Drugs in an Inner City Setting: An Observational Cohort Study. Subst. Use Misuse 2019, 54, 18–30.
- Graham, C.S.; Swan, T. A path to eradication of hepatitis C in low- and middle-income countries. Antivir. Res. 2015, 119, 89–96.
- Tordrup, D.; Hutin, Y.; Stenberg, K.; Lauer, J.A.; Hutton, D.W.; Toy, M.; Scott, N.; Bulterys, M.; Ball, A.; Hirnschall, G. Additional resource needs for viral hepatitis elimination through universal health coverage: Projections in 67 low-income and middle-income countries, 2016–2030. Lancet Glob. Health 2019, 7, e1180–e1188.
More
Information
Subjects:
Infectious Diseases
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
735
Revisions:
2 times
(View History)
Update Date:
05 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




