
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dejan Marčetić | + 1923 word(s) | 1923 | 2021-07-12 13:16:30 |
Video Upload Options
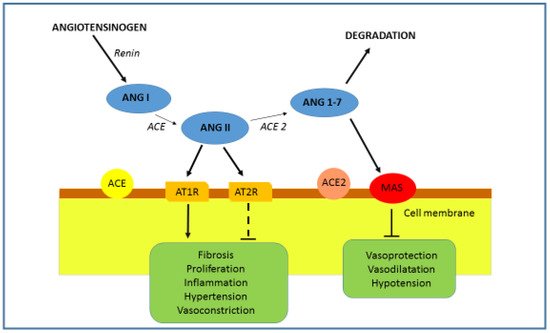
Angiotensin-converting enzyme 2 (ACE2) is a transmembrane glycoprotein discovered in the year 2000 [1,2]. ACE2 gene is located on the X chromosome (cytogenetic location: Xp22.2) and consists of 18 exons that encode for protein of 805 amino acids. ACE2 is a type 1 integral membrane glycoprotein with two domains, the amino-terminal catalytic domain and carboxy-terminal transmembrane domain. The active domain of ACE2 is exposed to the extracellular surface, facilitating the metabolism of circulating peptides. ACE2 is constitutively expressed by epithelial cells of the lungs—more precisely, on the surface of type I and type II alveolar epithelial cells . ACE2 is also expressed in the vascular system—endothelial cells, migratory angiogenic cells, and vascular smooth muscle cells. In the heart, ACE2 is expressed in the cardiomyocytes, cardiac fibroblasts, coronary vascular endothelium and epicardial adipose tissue. In the kidneys, ACE2 was detected in glomerular endothelial cells, podocytes and proximal tubule epithelial cells. ACE2 is also expressed and functional in the liver, enterocytes of the intestines, and the central nervous system . ACE2 is a component of the renin—angiotensin—aldosterone system (RAAS), a hormone system important in the regulation of blood pressure, fluid and electrolyte balance and the regulation of the systemic circulation . Abnormal activation of the RAAS has been associated with the pathogenesis of hypertension, heart failure and renal diseases. Its involvement in the inflammation pathogenesis is also well known .
1. Introduction
Chronic inflammatory lung diseases are characterized by uncontrolled immune response in the airways as their main pathophysiological manifestation. The lack of specific diagnostic and therapeutic biomarkers for many pulmonary diseases represents a major challenge for pulmonologists. The majority of the currently approved therapeutic approaches are focused on achieving disease remission, although there is no guarantee of complete recovery. It is known that angiotensin-converting enzyme 2 (ACE2), an important counter-regulatory component of the renin–angiotensin–aldosterone system (RAAS), is expressed in the airways. It has been shown that ACE2 plays a role in systemic regulation of the cardiovascular and renal systems, lungs and liver by acting on blood pressure, electrolyte balance control mechanisms and inflammation. Its protective role in the lungs has also been presented, but the exact pathophysiological mechanism of action is still elusive. The aim of this study is to review and discuss recent findings about ACE2, including its potential role in the pathophysiology of chronic inflammatory lung diseases:, i.e., chronic obstructive pulmonary disease, asthma, and pulmonary hypertension. Additionally, in the light of the coronavirus 2019 disease (COVID-19), we will discuss the role of ACE2 in the pathophysiology of this disease, mainly represented by different grades of pulmonary problems. We believe that these insights will open up new perspectives for the future use of ACE2 as a potential biomarker for early diagnosis and monitoring of chronic inflammatory lung diseases.
1.1. ACE2 and RAAS Regulation
1.2. ACE Inhibitors and ARBs
2. ACE2, Inflammation and Pulmonary Disease
3. Conclusions
References
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128.
- Tikellis, C.; Thomas, M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012, 2012, 256294.
- Hamming, I.; Cooper, M.E.; Haagmans, B.L.; Hooper, N.M.; Korstanje, R.; Osterhaus, A.D.M.E.; Timens, W.; Turner, A.J.; Navis, G.; van Goor, H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007, 212, 1–11.
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116.
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990.
- Li, C.; Bo, L.; Li, P.; Lu, X.; Li, W.; Pan, L.; Sun, Y.; Mu, D.; Liu, W.; Jin, F. Losartan, a selective antagonist of AT1 receptor, attenuates seawater inhalation induced lung injury via modulating JAK2/STATs and apoptosis in rat. Pulm. Pharmacol. Ther. 2017, 45, 69–79.
- Wang, R.; Zagariya, A.; Ibarra-sunga, O.; Gidea, C.; Ang, E.; Deshmukh, S.; Chaudhary, G.; Baraboutis, J.; Filippatos, G.; Uhal, B.D. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am. J. Physiol. 1999, 276, 885–889.
- Zhang, M.; Gao, Y.; Zhao, W.; Yu, G.; Jin, F. ACE-2/ANG1-7 ameliorates ER stress-induced apoptosis in seawater aspiration-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L1015–L1027.
- Martins, J.O.; Zanoni, F.L.; Martins, D.O.; Coimbra, R.; Krieger, J.E.; Jancar, S.; Sannomiya, P. Insulin regulates cytokines and intercellular adhesion molecule-1 gene expression through nuclear factor-κB activation in LPS-induced acute lung injury in rats. Shock 2009, 31, 404–409.
- Gao, Y.L.; Du, Y.; Zhang, C.; Cheng, C.; Yang, H.Y.; Jin, Y.F.; Duan, G.C.; Chen, S.Y. Role of renin-angiotensin system in acute lung injury caused by viral infection. Infect. Drug Resist. 2020, 13, 3715–3725.
- Santos, R.A.S.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The renin-angiotensin system: Going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H958–H970.
- Sarzani, R.; Giulietti, F.; Di Pentima, C.; Filipponi, A.; Spannella, F. Antagonizing the renin–angiotensin–aldosterone system in the era of COVID-19. Intern. Emerg. Med. 2020, 15, 885–887.
- Shen, L.; Mo, H.; Cai, L.; Kong, T.; Zheng, W.; Ye, J.; Qi, J.; Xiao, Z. Losartan prevents sepsis-induced acute lung injury and decreases activation of nuclear factorκB and mitogen-activated protein kinases. Shock 2009, 31, 500–506.
- Liu, L.; Qiu, H.B.; Yang, Y.; Wang, L.; Ding, H.M.; Li, H.P. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide-induced acute lung injury in rat. Arch. Biochem. Biophys. 2009, 481, 131–136.
- Jenkins, T.A.; Chai, S.Y. Effect of chronic angiotensin converting enzyme inhibition on spatial memory and anxiety-like behaviours in rats. Neurobiol. Learn. Mem. 2007, 87, 218–224.
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325.
- Brewster, U.C.; Perazella, M.A. The renin-angiotensin-aldosterone system and the kidney: Effects on kidney disease. Am. J. Med. 2004, 116, 263–272.
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 23, 7204–7218.
- Vandivier, R.W.; Henson, P.M.; Douglas, I.S. Burying the dead: The impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006, 129, 1673–1682.
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and inflammation in chronic respiratory disease. Autophagy 2018, 14, 221–232.
- Ferreira, A.J.; Shenoy, V.; Qi, Y.; Fraga-Silva, R.A.; Santos, R.A.S.; Katovich, M.J.; Raizada, M.K. Angiotensin-converting enzyme 2 activation protects against hypertension-induced cardiac fibrosis involving extracellular signal-regulated kinases. Exp. Physiol. 2011, 96, 287–294.
- Yamazato, Y.; Ferreira, A.J.; Hong, K.H.; Sriramula, S.; Francis, J.; Yamazato, M.; Yuan, L.; Bradford, C.N.; Shenoy, V.; Oh, S.P.; et al. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension 2009, 54, 365–371.
- Imai, Y.; Kuba, K.; Ohto-Nakanishi, T.; Penninger, J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010, 74, 405–410.
- Zou, Z.; Yan, Y.; Shu, Y.; Gao, R.; Sun, Y.; Li, X.; Ju, X.; Liang, Z.; Liu, Q.; Zhao, Y.; et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014, 5, 3594.
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326.
- Suski, M.; Olszanecki, R.; Stachowicz, A.; Madej, J.; Bujak-Gizycka, B.; Okoń, K.; Korbut, R. The influence of angiotensin-(1-7) Mas receptor agonist (AVE 0991) on mitochondrial proteome in kidneys of apoE knockout mice. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2463–2469.
- Zhong, J.; Basu, R.; Guo, D.; Chow, F.L.; Byrns, S.; Schuster, M.; Loibner, H.; Wang, X.H.; Penninger, J.M.; Kassiri, Z.; et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 2010, 122, 717–728.
- Dorsainval, W. ACE2/Ang1-7 MAS AXIS: The counter-regulator of the classical renin angiotensin system. Mako NSU Undergrad. Stud. J. 2020, 2020, 2.
- Gottschalk, G.; Knox, K.; Roy, A. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. Gene Rep. 2020, 23, 101077.
- Feng, Y.; Wan, H.; Liu, J.; Zhang, R.; Ma, Q.; Han, B.; Xiang, Y.; Che, J.; Cao, H.; Fei, X.; et al. The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol. Rep. 2010, 23, 941–948.




