1000/1000
Hot
Most Recent

Bioaerosol characterization represents a major challenge for the risk assessment and management of exposed people. One of the most important bioaerosol sources is the organic waste collection and treatment. This work analyzed and discussed the literature with the purpose of investigating the main techniques used nowadays for bioaerosol monitoring during organic waste treatment. The discussion includes an overview on the most effcient sampling, DNA extraction, and analysis methods, including both the cultural and the bio-molecular approach. Generally, an exhaustive biological risk assessment is not applied due to the organic waste heterogeneity, treatment complexity, and unknown aerosolized emission rate. However, the application of bio-molecular methods allows a better bioaerosol characterization, and it is desirable to be associated with standardized cultural methods. Risk assessment for organic waste workers generally includes the evaluation of the potential exposition to pathogens and opportunistic pathogens or to other microorganisms as biomarkers. In most cases, Saccharopolyspora rectivirgula, Legionella spp., Aspergillus spp., and Mycobacterium spp. are included. Future perspectives are focused on identifying common composting biomarkers, on investigating the causality process between chronic bioaerosol exposure and disease onset, and finally, on defining common exposure limits.
Bioaerosol characterization in organic waste treatment facilities is still a controversial topic, since the determination of the biological composition is strongly influenced by environmental factors, such as temperature, humidity, season, and prevalent source contamination, as well as technical factors like sampling and analysis methods. It is not yet possible to define a standardized method for bioaerosol characterization, although some identified procedures that can lead to a more homogeneous research and could allow a more exhaustive bioaerosol description. The critical points of the pipeline characterization are discussed here. Figure 1 reports a summary of the main preliminary steps to conduct a bioaerosol risk assessment.
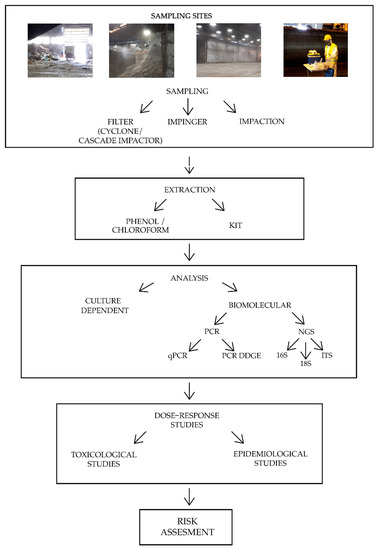
Figure 1. Summary of the main bioaerosol risk assessment steps. The four pictures at the top represent common sampling sites in organic waste treatment plants. Note: PCR = Polymerase Chain Reaction; qPCR = quantitative Polymerase Chain Reaction; PCR DGGE = Polymerase Chain Reaction Denaturing Gradient Gel Electrophoresis; NGS = Next Generation Sequencing; 16 S = ribosomal region 16S of bacteria; 18 S = ribosomal region 18S of fungi; ITS = Internal Transcribed Spacer.
Results from exposure studies are extremely influenced by the sampling method. In the last decade, the number of samplers for bioaerosol has increased. Sampling methods commonly used and regulated by international standardized operative procedures are filtration, impaction, liquid impinger, and cyclone[1][2][3].
Filtration consists of pumping air through a porous membrane filter that captures bioaerosol particles. This method can be used both in culture-dependent and culture-independent studies. This sampler is cheap, easy to use, efficient at trapping microorganisms larger than a pore size, and the captured microorganisms remain viable[4]. Samples could be subjected to desiccation due to the flow rate and time; therefore, the spore-forming microorganisms may be preferentially recovered[5]. Generally, a personal sampler runs at a low-flow sampling rate, and only a small volume of air can be sampled on the filter. The sample of bioaerosol is not concentrated enough for an exhaustive evaluation; therefore, the environmental sampling can supply another element for risk assessment[6][7].
Impaction consists in the use of an air pump that captures the air over the surface of a Petri dish containing nutrient agar. Fungal and bacterial samples can be used for culture-dependent studies; it is also easy to use and cheap. The disadvantages are loss of viability and loss of recovery efficiency due to the lack of adherence to the surface by the microorganisms[4].
Liquid impinger captures bioaerosol in a liquid matrix. Samples can be analyzed with culture-dependent and -independent methods. These samplers are made of glass, and they can be easily broken, meaning that they are not easy to use, and they are expensive. Samples could be subjected to evaporation and violent bubbling, which could lead to a loss of sample[5].
Cyclone captures bioaerosol into a liquid matrix using centrifugal force. Samples can be analyzed with culture-dependent and -independent techniques. As observed for the impinger method, one of the main disadvantages is the possible evaporation of the liquid medium and the subsequent loss of sample. Another one is the low quantity of sample collected. Moreover, larger molecules are preferentially collected. This sampler is easy to sterilize[7][8].
Nowadays, researchers are trying to improve the real-time bioaerosol monitoring. The currently used devices only count particles but are not able to classify them. The last improvement of such technique included biochemical determination to detect biological components even if there are a lot of non-biological interferents[9]. The ideal real-time sampler should be portable, have continuous sampling and high collection efficiency, and should continuously deliver samples to the detection system. The slow development in this field is due to the difficulty in determining the concentration and the type of bioaerosols in the air stream. One of the main disadvantages is the misinterpretation due to the signal interference from non-biological particles[10].
The first phase of this study was focused on the comparison of the commonly used sampling methods (Table 1, first section referring to sampling methods), tested both on samples generated in lab and on samples from a composting site. Ferguson et al. observed that Filtration with polycarbonate (PC) filters produces better results in terms of bacterial DNA recovery than Liquid impinger with a difference of an order of magnitude[11]. This is probably due to the easier extraction of the sample from the surface of the filter or to the recovery of particles with a diameter size of less than 0.5 µm, which results extremely difficult with the impinger method. Furthermore, filters in PC are thin; hence, they completely dissolve in phenol/chloroform solution making the extraction lysis process more efficient[11]. Despite the higher DNA extraction, in filtration, the bacteria quantification was scarce and the quality of the DNA was low compared to the Impaction method (Low Melting Agar)[11]. This could be due to the not optimal extraction methods or to the strong dehydration during high flow sampling rate. It is also important to define an ideal flow rate and sampling time in order to reduce the sample loss due to the desiccation. On the other hand, too short sampling times and low flow sampling rates produce an insufficient collection of bioaerosol for proper extraction and analysis. The use of an optimized extraction kit could make this process easier, time-saving, and would also allow the use of a standardized extraction method common for all laboratories[12][13][14].
Table 1. Sampling, extraction and analysis methods summary. Note: PCR = Polymerase Chain Reaction; qPCR = quantitative Polymerase Chain Reaction; DGGE = Denaturing Gradient Gel Electrophoresis; NGS = Next Generation Sequencing; MALDI-TOF = Matrix-assisted laser desorption/ionization time of flight. [5][11][15][14][16][17][18][19][20][21][22][23][24][25][26][27][28]
| Methods | Strength | Weakness | |
|---|---|---|---|
| Sampling | Filtration | High collection efficiency (for particles >0.4 μm); Portability; Viable microorganisms; Cultural and biomolecular analysis Variable flow rates, it depends on the employed sampler (low 2 L/min; medium 10–30 L/min or high 300–1000 L/min) Also virus collection |
Spore forming microorganism’s selection; Dehydrating problem at high flow rate The low and medium flow produce a scarce quantity of material for the bioaerosol analysis |
| Impaction | Variable flow rate but generally <200 L/min; Portability; Generally, only cultural method |
Difficult nucleic acids extraction; Viability reduction; Recovery reduction |
|
| Impinger | Low flow rates (<100 L/min); Shorter sampling period; Cultural and biomolecular analysis; Sample directly in liquid |
Lower collection efficiency than filters; Low portability; Difficult nucleic acids extraction; Evaporation bias Less cheap |
|
| Extraction | Phenol-chloroform | Better lysis Cheap |
Not standardized Time-consuming |
| Commercial kits (DNeasy PowerSoil Kit Qiagen, PowerViral DNA/RNA Kit Qiagen, etc.) |
Standardized method Time-saving |
Less cheap | |
| Current Analysis | Cultural | Viable microorganisms; Cheap; Possible API® determination |
Qualitative analysis; Do not detect unculturable microorganisms; Detection of 1.5–15.3% of all the species |
| Real time qPCR | Quantitative analysis; High sensitivity and accuracy; High reproducibility; High number of samples; Detect unculturable microorganisms |
Detect also non-viable microorganisms | |
| PCR-DGGE | Numerically dominant community members; Community changes and differences |
Weak reproducibility; Low specificity; Qualitative analysis; Gradient production |
|
| MALDI-TOF | Rapid analysis after the growth on plate Sensitive Cheap if the equipment is already available |
Previous cultural step or bioaerosol sample pre-treatment; Identification limited to known peptide mass fingerprints |
|
| NGS Analysis | Targeted Amplicon Sequencing rRNA | Bacterial and fungal sequencing; Reduction of bias with degenerated oligonucleotides; Most used method |
High sample quantity; Previous PCR step; Expensive; Over-estimation; Detect non-viable microorganisms; Relative abundance sloped; No viral identification |
| Shotgun metagenomics | Whole genome Viral detection Taxonomic biodiversity and biological functions Global biome characterization Absolute abundance No PCR biases No previous knowledge of the sequences |
High sample quantity; Expensive; Challenging bioinformatics analyses; Detect non-viable microorganisms |
The commonly used methods for bioaerosol characterization are culture-dependent and quantitative Polymerase Chain Reaction (qPCR) techniques. Moreover, some studies included Next Generation Sequencing (NGS) of the regions 16S and 18S of rRNA or rDNA (Table 1).
These three methods do not exclude each other; in fact, the use of all of them allows to increase the comprehension of the bioaerosol composition. The cultural method only recognizes 1.5–15.3% of all the species that are able to create a colony, and all the microorganisms that are not viable or unable to grow are not identified[29]. In addition, a metagenomic approach could be theoretically applicable; nowadays, there are no published papers where the metagenomic method was applied to bioaerosol composting samples. Such approach could provide a more exhaustive microbiota characterization including also fungi and viruses, but on the other hand, it is still expensive. Traditional culture-dependent studies require selective culture media that allow the growth of target microorganisms limiting the undesired ones, but in the practice, the biodiversity of the matrix highlights overlapping and unexpected growth.
Bacterial and fungal quantifications are a preliminary good contamination index[29]. As previously stated, various specific targets have been identified such as total bacteria, Legionella pneumophila, Saccharopolyspora rectivirgula[30], and Thermoactinomyces vulgaris[27][31] . These targets were used to compare culture-dependent and quantitative PCR. Betelli et al. observed that there is a linear correlation between the copy number of the region 16S calculated with the qPCR and the number of CFU observed with the cultural method[32]. The culture-independent methods allow to assess the concentration of microorganisms 2 or 3 orders of magnitude higher than the culture-dependent techniques. Indeed, the cultural method estimates only about 15% of the bacteria quantification from Low Melting Agar (LMA) plates studied with qPCR[33] . Therefore, biomolecular methods seem to be more efficient than culture-dependent ones. On the contrary, Shade et al. determined the presence of a fraction of microorganisms and very low concentrations only found with the culture-dependent method[34]. The culture-independent methods allow instead to determine a concentration hundred or thousand times higher than the culture-dependent method. It also allows the identification of a large part of the microorganisms present in the sample, but it does not allow to discern the microorganism’s viability. With the Polymerase Chain Reaction (PCR), a given microorganism is identified through the amplification of a specific region of its nucleic acid. The qPCR technique, based on the measurement of the emitted fluorescence from a target during the amplification process, could also be used for a quantitative result[24].
A major disadvantage of qPCR is that it does not determine cell viability. However, a viability assay that combines qPCR with propidium monoazide (PMA-qPCR) can set alive and dead cells apart. Propidium monoazide, as well as ethidium monoazide, is a DNA dye able to bind free DNA avoiding the subsequent PCR reaction[35]. For this reason, PMA-qPCR was proposed to assess the feasibility of detecting the viability in various samples. The application on bioaerosol is very limited, but the preliminary results showed the general feasibility of PMA-qPCR in the aerobiology and the presence of a high quantity of viable but not cultivable bacteria in the air[36].
The Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) is not commonly used, and it is typically replaced by Next Generation Sequencing (NGS) techniques. However, it allows a primary investigation of the numerically dominant community members, community changes, and differences. It has a scarce reproducibility and sensitivity though.
NGS is one of the latest technological innovations introduced in the biomolecular practice. The main techniques used are 454 pyrosequencing and Illumina.
Pyrosequencing is based on an emulsion PCR on microbeads. Each bead covered with DNA amplicons is contained in a plot slightly larger than the beads. On the bottom of the support there is a light detector[37]. Illumina is based on bridge PCR on a glass surface, that increases both the density and number of DNAs that can be monitored at the same time[5][38]. Typically, the sequencing for bioaerosol characterization is based on the analysis of the region 16S or 18S of the rRNA for bacteria and fungi, respectively. Moreover, the ribosomal ITS (Internal Transcribed Spacer) is used as a universal marker for fungal characterization. Viruses do not share conserved genetic regions, and the virome composition is still largely undefined[23][39]. In databases like SILVA—exclusively dedicated to the collection of specific DNA regions—there are millions of full-length sequences of the genes that encode for the region 16S of the 30S subunit of the rRNA. The sequencing of the 16S is based on the study of highly variable regions (V1-V9) with particular attention to the V4-V5 regions, which encode for bacteria[23]. Moreover, it is also possible to conduct whole genome sequencing, allowing a global analysis of the genome sample. It requires high sample quantities, it is expensive, and the bioinformatic analyses are still challenging. On the other hand, it allows a global microbiome characterization (e.g., virome), including taxonomic biodiversity and biological functions information.
Few metagenomic studies on bioaerosol were published. The collection of adequate biomass is crucial for successful metagenomic analysis. Recently, metagenomic analysis of the airborne DNA and RNA were performed in a daycare center, starting from the filters of the ventilation and the air conditioning system[40]. Other studies on bioaerosol composition focused on influenza circulation, they extracted RNA from samples collected with different methods, and then they evaluated the samples with RT-PCR. The results showed viral RNA detection rates >70% but also heterogeneity between the different methods used[41]. In the waste treatment, the sample size is a limiting factor, and the published studies mainly showed only a greater complexity of the dispersed microbiota[30].
The cultural method, especially if preceded by an enrichment phase, is able to identify microorganisms that are present in the environment at very low concentrations, thus allowing the tracing of potentially pathogenic microorganisms. A culture-dependent confirmation and typing with culture-independent techniques are necessary to establish a causal link between the infectious episode and the exposure. The analysis method is truly complete only when both the biomolecular and cultural studies are combined[42].
Matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry has been proposed for microbial identification and diagnosis. This technology has some advantages because it is rapid, sensitive, and relatively economical. Nevertheless, this method has also some limitations. For example, the identification of new isolates is possible only if the spectral database contains peptide mass fingerprints of specific genera/species/subspecies/strains[18]. This is limiting especially for the bioaerosol characterization. In addition, a previous cultural phase on a plate or a pre-treatment phase of the bioaerosol sample is needed[22]. However, MALDI-TOF mass spectrometry has been already used to characterize waste workers’ exposure to bioaerosols during waste collection[21] and for the evaluation of bacterial and fungal species to assess the biological risk which waste collection workers’ are exposed to[16]. MALDI-TOF was also employed to increase our knowledge of the physicochemical and biological characteristics of bioaerosols from composting sites[17].
To perform risk assessments, some scientists decided to investigate the presence of harmful microorganisms for human health using qPCR. Dubuis et al. selected four bacterial biomarkers that have been shown in composting facilities in order to verify their presence also in biomethanization areas: Saccharopolyspora rectivirgula, Legionella spp., Legionella pneumophila, and Mycobacterium spp.[30]. S. rectivirgula is correlated with the onset of the Farmer’s lung disease[29][43]; L. pneumophila causes a serious disease, the Legionnaires’ disease[44]; Mycobacterium spp. can cause bone, skin, and lung infections.
Mbareche et al. focused their attention on the study of fungal composition of bioaerosols emitted from biomethanization sites and decided to use Aspergillus spp. and Aspergillus fumigatus as bioindicators .
The investigation of A. fumigatus is important due to its correlation with pulmonary diseases such as aspergillosis[45].
Moreover, Traversi et al. used specific bioindicators such as Pseudomonaceae to improve the risk assessment in biomethanization plants[6]. Indeed, Pseudomonas is commonly associated with pneumonia and osteoarticular infections[46][47], and it is a biofilm bioindicator, especially in water systems[6].
Nowadays, an exhaustive biological risk assessment is weakly applied because it is strongly influenced by parameters that are difficult to estimate and that are often arbitrarily defined, such as the variability of microorganisms present in the bioaerosol, the rate of bioaerosol released from the matrix, the inter-individual variability of the exposed subjects, etc. In addition, the lack of validated methodology causes a deformity in the evaluation that makes the results incomparable.
Mbareche et al. suggested the creation of a worldwide database containing all the sequences obtained from the sequencing analysis with the description of the sampling and analysis methods used. This would allow a definition of the microbial composition of bioaerosol[48].
The sampling method based on filters is currently the most satisfying, but it requires improvement and standardization of the extraction methods. The biomolecular analysis allows a better bioaerosol characterization, but this technique does not distinguish between viable or non-viable microorganisms, potentially leading to an overestimation. Another important issue for qPCR application is that microorganisms could have more than one copy of the region 16S, as previously demonstrated in the literature[30]. Therefore, the data elaboration has to take into account such evidence whenever there are quantitative purposes.