
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Johannes Hans Crezee | + 2452 word(s) | 2452 | 2021-07-14 08:13:38 | | | |
| 2 | Amina Yu | Meta information modification | 2452 | 2021-07-19 03:17:43 | | |
Video Upload Options
The concept of HIPEC is to penetrate and eradicate potential small tumor nodules at the peritoneal surface with a sufficient cytotoxic amount of chemotherapy. Preclinical in vivo HIPEC models should mimic realistic clinical HIPEC conditions including a relevant and realistic tumor model, well-controlled drug, flow and temperature distribution within the peritoneal cavity. Advantages and limitations of different tumor and animal models as well as model set up are discussed.
1. Introduction
The relevance of a careful choice of treatment parameters was recently underscored by the outcome of the PRODIGE-7 trial in which patients were treated with high-dose oxaliplatin by the closed (360 mg/m2) or open (460 mg/m2) delivery technique at a relatively high temperature (43 °C) for a short (30 min) duration [1]. In a comment, Ceelen argued that the choice of chemotherapy, treatment duration, carrier solution, and treatment temperature could have negatively impacted trial outcomes [2]. Not all colorectal cancer subtypes respond similarly to oxaliplatin and the treatment duration was not optimal to maximize the effect of oxaliplatin exposure [3]. This clinical evidence is vital for successful application of HIPEC and therefore, these study protocols should be designed to be able to provide solid recommendations on optimal treatment.
Strong scientific evidence for the selection of treatment parameters is essential to optimize treatment protocols and design successful clinical trials. The efficacy of the HIPEC procedure may be influenced by patient selection, delivery technique, and treatment parameters, including temperature, carrier solution, type of drug, dosage, volume, and treatment duration [4]. Lack of sufficient data may explain why the choice of these parameters varies significantly among HIPEC experts around the world [5]. Therefore, solid scientific evidence is needed to support an optimal choice of treatment parameters.
Several factors associated with treatment effectiveness have already been evaluated in clinical studies. Spielberg et al. showed in another retrospective study in patients suffering from PM of colorectal cancer origin that the use of oxaliplatin resulted more frequently in postoperative complications compared to MMC [6]. A study in gastric cancer patients showed that complete CRS is important for survival [7], while lymph node involvement has a negative effect on progression-free survival [8]. Fagotti et al. showed that recurrent ovarian cancer patients could be safely treated with minimally invasive surgery in combination with cisplatin- or oxaliplatin-based HIPEC, showing promising results [9][10].
Preclinical research offers a powerful tool for investigating the impact of HIPEC treatment parameters on treatment outcomes. Data provided by in vitro research can provide interesting insights into the molecular effect of various chemotherapeutic agents on tumor and normal tissue cell lines at different, well-controlled, temperatures [11][12][13]. Adequate in vivo models thus remain essential for the successful translation of preclinical results into relevant clinical protocols. Furthermore, it should be possible to vary individual treatment parameters while keeping other parameters constant to allow differentiation between the effects of different treatment parameters on the efficacy of HIPEC.
There are many general HIPEC review papers in which also preclinical models are discussed [14][15][16][17][18][19], but so far, no comprehensive review discussing these preclinical in vivo models for HIPEC is available, and such a specific review will assist in making optimal model choices for in vivo research. This study reviews the objectives, methods, advantages, and limitations of preclinical animal models used for research on relevant in vivo HIPEC treatment parameters relevant for treatment outcome. Relevant features and challenges of preclinical animal models, the HIPEC setup, and research categories are discussed (Figure 1). Finally, guidelines are provided on how to design and develop models specifically aiming at certain parameters.
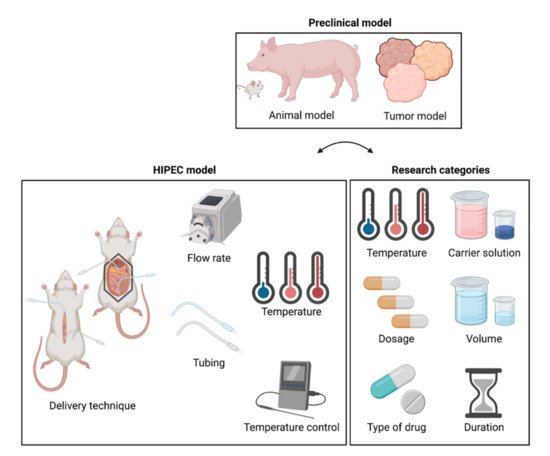
2. HIPEC Research Categories
The 60 in vivo HIPEC research papers discussed in this review investigated a wide range of research questions. These research questions are organized into research categories (see Table 1). We discuss the studies performed per research category, possible future experiments essential for optimizing HIPEC, what is needed to ensure adequate performance, and possible pitfalls. 1the research categories, the number of studies in each research category, and recommendations for tumor and animal models for future studies are presented.
| Research Category Effect of: | Number of Papers | Reference(s) | Study Goal | Recommended Animal Model | Recommended Tumor Model |
|---|---|---|---|---|---|
| 1. Type of drug | 15 | [20][21][22][23][24][25][26][27][28][29][30][31][32][33][34] | Uptake and/or sensitivity of tumor tissue | Mouse, rat | Syngeneic, xenograft or PDX |
| Immune response | Mouse, rat | Syngeneic | |||
| 2. Drug concentration | 11 | [20][24][28][35][36][37][38][39][40][41][42] | Uptake by ‘healthy’ organs | Pig | Not required |
| Uptake and/or sensitivity of tumor tissue | Mouse, rat | Syngeneic, xenograft or PDX | |||
| 3. Carrier solution | 1 | [31] | Drug effectiveness | Mouse, rat | Syngeneic, xenograft or PDX |
| Systemic toxicity | Pig | Not required | |||
| 4. Volume | 1 | [43] | Drug and temperature distribution | Pig | Not required |
| Drug effectiveness | Mouse, rat | Syngeneic, xenograft or PDX | |||
| 5. Temperature | 2 | [39][40] | Effect on drug uptake and/or tumor sensitivity | Mouse, rat | Syngeneic, xenograft or PDX |
| Systemic toxicity | Pig | Not required | |||
| 6. Duration | 1 | [43] | Uptake and/or sensitivity of tumor tissue | Mouse, rat | Syngeneic, xenograft or PDX |
| Systemic toxicity | Pig | Not required | |||
| 7. Delivery technique | 6 | [44][45][46][47][48][49] | Drug and temperature distribution in the peritoneal area | Pig | Not required |
| Systemic toxicity | Pig | Not required |
3. Preclinical HIPEC Models
Important parameters of preclinical HIPEC research models to study physiological effects and anticancer activity include a selection of a suitable animal model, tumor model, and setup. We can distinguish two types of models: physiological models, which determine for example tolerable temperatures and volumes to establish whether a drug can be given in the peritoneum, and anticancer models to evaluate the expected level of anticancer activity. The selection of relevant parameters depends on the research category and study goals. Considerations for proper selection are discussed in separate sections for each of these research model parameters, followed by sections on each of the research categories.
Several types of animals have been used as a preclinical model to study HIPEC in the analyzed articles. Most often rats (47%) were used, followed by mice (27%), pigs (22%) and rabbits (5%). Depending on the origin of the used cancer cells, rat and mouse strains were chosen. For mouse experiments, the following strains were used: Swiss albino (20%) [24][26][50], athymic (33%) [25][36][38][43][51], C57BL/6 (20%) [21][27][52], BALB/c (20%) [37][44][53] and NOD-SCID (7%) [22].
The main advantage of using small animals in preclinical HIPEC studies is the fact that one can relatively easily establish PM from a variety of origins. The main drawback of using small animals is the significantly smaller peritoneal cavity compared to a human’s. In general, small animal models can only be used to study the local effects of HIPEC and are most suitable to study anticancer effects such as the tumor penetration depth, tumor biology, tumor sensitivity, and tumor survival. The small flow region is also not ideally suited to test the optimal clinical flow setup where thermal and drug distributions can vary widely between flow region sizes.
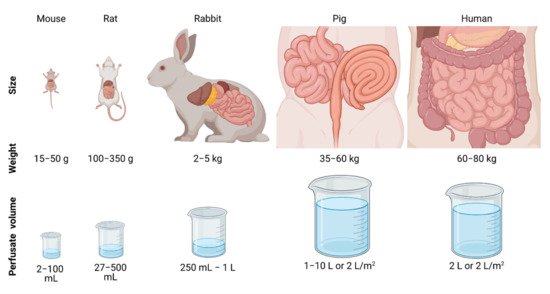
Large animals, such as pigs, were less frequently used as a preclinical model in HIPEC research. Lack of good tumor models in pigs, i.e., development of tumor lesions in the peritoneal area, hamper evaluation of the effect of HIPEC effects on tumor size or survival. Nevertheless, larger animal models are ideal for studying the macroscopic and systemic effects of HIPEC, because both size and anatomy are comparable to the human peritoneum, see Figure 2. Determining these factors in smaller animals is also possible, but the clinical translation would be more complex, and therefore, larger, preferably more human-size animals are preferred.
PM were induced in most of the reviewed articles (52%), while 48% of the studies performed HIPEC in animals when no PM were induced. In the included articles, PM was induced in rats (46%), mice (100%) and rabbits (66%) before HIPEC treatment. [20][21][23][24][26][27][29][30][32][33][35][37][42][44][54][55][56][57][58][59][50], whereas xenograft (28%) [22][25][36][38][43][60][51][52] and patient derived xenograft (PDX) (3%) Tumors were established from different cell lines originating from colorectal (45%)
Animals were injected with cancer cells, or cancer tissue was transplanted into the peritoneal area to develop small tumor lesions throughout the abdominal cavity. Syngeneic models are immunocompetent animals that receive cancer cells or tissue derived from the same genetic background [61]. However, syngeneic models do not replicate the molecular background of human cancers. PDX models are immunodeficient animals in which patient-derived cancer cells or tissues are grafted to overcome the loss of genetic and morphological heterogeneity of the xenograft model.
Each tumor model has its advantages which should be taken into account. All tumor models can be used to study the anticancer activity such as tumor penetration depth of the drug, but xenograft and PDX models should be used to study the tumor biology, excluding immune-related phenomena, and tumor sensitivity. When applying immunotherapy or studying the immune response, the syngeneic model is a logical choice.
Studies in which PM was successfully developed used different cell lines, cell amount, and dissolvent, resulting in the corresponding tumor take rates, tumor outgrowth time, PCI score, and tumor locations. Overall, the tumor implementation-take rate was 64–100%, with tumor formation seen from 2–27 days after injection, and a PCI score of 2–20, depending on the tumor model. In rats, the colorectal cancer cell line CC531 was most often used, with a tumor take rate of 100%, tumor outgrowth seen at 7–8 days after injection, resulting in tumor nodules mainly present on the greater omentum, liver hilum, perisplenic area and mesentery [35][42][55][56][57][58][59]. High-resolution pictures showing the spread of the tumor nodules in the peritoneum and a proper scoring (e.g., PCI score) are often missing.
The concept of HIPEC is to penetrate any potential tumor nodules with a sufficient amount of chemotherapy anywhere at the peritoneal surface, which requires an equal distribution of the perfusate with the same temperature and concentration. Controlling and stabilizing the thermal profile is a difficult task. This was underlined by a study that measured thermal profiles in rats during a semi-open HIPEC, showing that fluctuations of a few degrees are not uncommon [62]. Three flow parameters can be adjusted to optimize the thermal homogeneity during open and close HIPEC treatments: flow rate, number of catheters, and catheter placement.
The flow rate can impact both the heating rate and homogeneity during treatment [62]. The ratio of the heat applied and the cooling rate is therefore proportional to the length scale, making it relatively more difficult to reach the required treatment temperature within the peritoneal cavity. Doubling the flow rate increased the thermal homogeneity while halving the flow rate resulted in increased thermal heterogeneity [63][62]. Maximizing flow rates, while maintaining a tolerable core temperature should be preferred.
It was not possible to correlate the effect of the flow rate with the intra-abdominal temperature since few studies included thermal measurements (33%). Higher flow rates increase the heat flux into the system of the animal, raising the core temperature. Studies using a flow rate of 10 milliliters per second (n = 4) reported core temperatures ranging from 35–37 °C. All studies using higher flow rates (n = 3) reported core temperatures above 37 °C.
Elevated temperature can have a profound effect on the efficacy of the chemotherapy used. Therefore, if no thermal homogeneity is achieved, it will become difficult to fairly assess the effect of different choices in all other research parameters. Thermal measurements are also crucial for assuring thermal homogeneity. Future experiments could determine the minimal amount of probes required for different animals.
Elevated peritoneal temperatures can result in systemic overheating of the animal. Therefore, it is important to monitor the core temperature during treatment and to prevent overheating by cooling if necessary. In almost all cases the rectal temperature was used as a reference, sometimes the temperature under the tongue was measured. Rectal temperatures varying from 32–39 °C were observed in rats, mice, and pigs, whereas 37.5–38 °C was measured under the tongue of rats.
The heat sink effects vary between differently sized animals, and therefore the heating and cooling procedure should be adapted to animal size. For these animals, it is extremely important to carefully monitor the core temperature during the entire surgical procedure and to start heating or cooling in time upon temperature change. In larger animals, the core temperature is more stable, and therefore extensive heating or cooling is usually not required. When core temperature profiles from preclinical studies need to reflect the human clinical situation, larger animals with a representative size to humans should be used, e.g., pigs.
Furthermore, the location where the core temperature is measured is crucial. Currently, no studies are comparing different locations to measure the core temperatures, but rectal temperature measurements are likely not adequate enough since the placement of the probe is close to the area perfused by HIPEC and rectal temperatures, therefore, tend to rise rapidly. Preferably, core temperatures should be monitored further away from the perfused area, e.g., underneath the tongue or ideally in the esophagus. To further investigate the effect of elevated temperature on the systemic level, it is necessary that reporting the core temperature profile during HIPEC becomes standard.
4. Outlook
Positive outcomes during animal studies can be very helpful to design clinical (feasibility) studies to further optimize clinical treatments. The clinical interpretation of animal study results is strongly dependent on model and HIPEC parameters used and therefore careful study design parameter selection is very important. Even with those precautions, preclinical tumor models have limitations in how well they can represent the response in clinical application in humans. In this article, we broke down HIPEC research into several research categories that can be translated to clinically relevant treatment parameters.
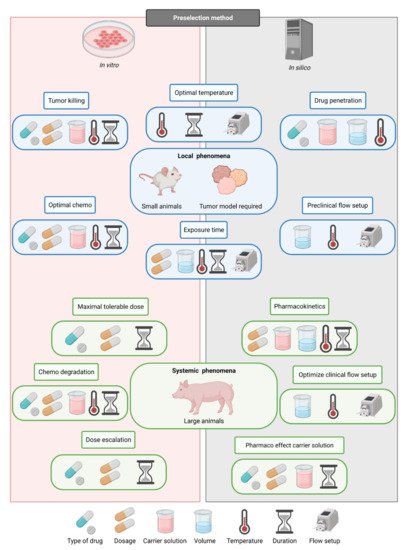
First and foremost, the delivery of HIPEC, specifically the thermal and drug distributions, should be well-controlled. If there are unknown large temperature variations in treatment delivery in and between animals, it is impossible to make a fair comparison between different choices composing the HIPEC treatment. Flow optimization should be performed for experimental setups to reduce these variations. Variations will persist, but these variations have to be quantified such that they can be taken into account while analyzing experimental results.
When the control and uniformity of the treatment can be ensured, other parameters can be compared. On the left side of the figure, it is indicated if certain study goals need in vitro or in silico preselection of HIPEC research categories. It is very important to decide on the correct order in which the parameters should be determined since most parameters are cross-dependent, as is visible in Figure 3. This way, future preclinical studies provide strong guidance toward optimal HIPEC protocols, which can subsequently be tested in a clinical setting.
References
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266.
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. (EJSO) 2019, 45, 400–402.
- Kirstein, M.N.; Root, S.A.; Moore, M.M.; Wieman, K.M.; Williams, B.W.; Jacobson, P.A.; Marker, P.H.; Tuttle, T. Exposure–response relationships for oxaliplatin-treated colon cancer cells. Anti-Cancer Drugs 2008, 19, 37–44.
- Helderman, R.F.C.P.A.; Löke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.A.P.K.; Crezee, J. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers 2019, 11, 78.
- Bushati, M.; Rovers, K.; Sommariva, A.; Sugarbaker, P.; Morris, D.; Yonemura, Y.; Quadros, C.; Somashekhar, S.; Ceelen, W.; Dubé, P.; et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1942–1948.
- Spiegelberg, J.; Neeff, H.; Holzner, P.; Runkel, M.; Fichtner-Feigl, S.; Glatz, T. Comparison of hyperthermic intraperitoneal chemotherapy regimens for treatment of peritoneal-metastasized colorectal cancer. World J. Gastrointest. Oncol. 2020, 12, 903–917.
- Ji, Z.-H.; Yu, Y.; Liu, G.; Zhang, Y.-B.; An, S.-L.; Li, B.; Li, X.-B.; Yan, G.-J.; Li, Y. Peritoneal cancer index (PCI) based patient selecting strategy for complete cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy in gastric cancer with peritoneal metastasis: A single-center retrospective analysis of 125 patients. Eur. J. Surg. Oncol. (EJSO) 2021, 47, 1411–1419.
- Baumgartner, J.M.; Tobin, L.; Heavey, S.F.; Kelly, K.J.; Roeland, E.J.; Lowy, A.M. Predictors of Progression in High-Grade Appendiceal or Colorectal Peritoneal Carcinomatosis after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2014, 22, 1716–1721.
- Fagotti, A.; Petrillo, M.; Costantini, B.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Turco, L.C.; Bottoni, C.; Scambia, G. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: A case series. Gynecol. Oncol. 2014, 132, 303–306.
- Gallotta, V.; Fagotti, A.; Fanfani, F.; Ferrandina, M.G.; Nero, C.; Costantini, B.; Alletti, S.G.; Chiantera, V.; Ercoli, A.; Scambia, G. Laparoscopic surgical management of localized recurrent ovarian cancer: A single-institution experience. Surg. Endosc. 2014, 28, 1808–1815.
- Urano, M.; Ling, C.C. Thermal enhancement of melphalan and oxaliplatin cytotoxicity in vitro. Int. J. Hyperthermia 2002, 18, 307–315.
- Helderman, R.F.C.P.A.; Löke, D.R.; Verhoeff, J.; Rodermond, H.M.; van Bochove, G.G.; Boon, M.; van Kesteren, S.; Vallejo, J.J.G.; Kok, H.P.; Tanis, P.J.; et al. The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines. Cells 2020, 9, 1775.
- Murata, S.; Yamamoto, H.; Shimizu, T.; Naitoh, H.; Yamaguchi, T.; Kaida, S.; Takebayashi, K.; Miyake, T.; Tani, T.; Tani, M. 5-fluorouracil combined with cisplatin and mitomycin C as an optimized regimen for hyperthermic intraperitoneal chemotherapy in gastric cancer. J. Surg. Oncol. 2018, 117, 671–677.
- Ceelen, W.; Demuytere, J.; de Hingh, I. Hyperthermic Intraperitoneal Chemotherapy: A Critical Review. Cancers 2021, 13, 3114.
- Bhatt, A.; de Hingh, I.; van der Speeten, K.; Hubner, M.; Deraco, M.; Bakrin, N.; Villeneuve, L.; Kusamura, S.; Glehen, O. HIPEC Methodology and Regimens: The Need for an Expert Consensus. Ann. Surg. Oncol. 2021, 1–16.
- Bakkers, C.; Simkens, G.A.A.M.; de Hingh, I.H.J.T. Systemic therapy in addition to cytoreduction and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: Recent insights from clinical studies and translational research. J. Gastrointest. Oncol. 2021, 12, S206–S213.
- Van Stein, R.M.; Aalbers, A.G.J.; Sonke, G.S.; van Driel, W.J. Hyperthermic Intraperitoneal Chemotherapy for Ovarian and Colorectal Cancer. JAMA Oncol. 2021.
- Kranenburg, O.; van der Speeten, K.; de Hingh, I. Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges. Front. Oncol. 2021, 11, 650098.
- Ceelen, W.; Braet, H.; van Ramshorst, G.; Willaert, W.; Remaut, K. Intraperitoneal chemotherapy for peritoneal metastases: An expert opinion. Expert Opin. Drug Deliv. 2020, 17, 511–522.
- Bespalov, V.G.; Alvovsky, I.; Tochilnikov, G.V.; Stukov, A.N.; Vyshinskaya, E.A.; Semenov, A.L.; Vasilyeva, I.N.; Belyaeva, O.A.; Kireeva, G.S.; Senchik, K.Y.; et al. Comparative efficacy evaluation of catheter intraperitoneal chemotherapy, normothermic and hyperthermic chemoperfusion in a rat model of ascitic ovarian cancer. Int. J. Hyperth. 2017, 34, 545–550.
- Huang, W.-C.; Wu, C.-C.; Hsu, Y.-T.; Chang, C.-L. Effect of hyperthermia on improving neutrophil restoration after intraperitoneal chemotherapy. Int. J. Hyperth. 2019, 36, 1254–1262.
- Graziosi, L.; Mencarelli, A.; Renga, B.; Santorelli, C.; Cantarella, F.; Bugiantella, W.; Cavazzoni, E.; Donini, A.; Fiorucci, S. Gene expression changes induced by HIPEC in a murine model of gastric cancer. Vivo 2012, 26, 39–45.
- Bevanda, M.; Orsolic, N.; Basic, I.; Vukojević, K.; Benkovic, V.; Knezevic, A.H.; Lisičić, D.; Dikic, D.; Kujundžić, M. Prevention of peritoneal carcinomatosis in mice with combination hyperthermal intraperitoneal chemotherapy and IL-2. Int. J. Hyperth. 2009, 25, 132–140.
- Oršolić, N.; Car, N. Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumor Biol. 2014, 35, 6445–6454.
- Muenyi, C.S.; States, V.; Masters, J.H.; Fan, T.W.; Helm, C.W.; States, J.C. Sodium arsenite and hyperthermia modulate cisplatin-DNA damage responses and enhance platinum accumulation in murine metastatic ovarian cancer xenograft after hyperthermic intraperitoneal chemotherapy (HIPEC). J. Ovarian Res. 2011, 4, 9.
- Düzgün, Ö.; Sarici, I.S.; Gokcay, S.; Ates, K.E.; Yılmaz, M.B. Effects of nivolumab in peritoneal carcinamatosis of malign melanoma in mouse model. Acta Cir. Bras. 2017, 32, 1006–1012.
- Lehmann, K.; Rickenbacher, A.; Jang, J.-H.; Oberkofler, C.E.; Vonlanthen, R.; von Boehmer, L.; Humar, B.; Graf, R.; Gertsch, P.; Clavien, P.-A. New Insight into Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. 2012, 256, 730–738.
- Trépanier, J.-S.; Sideris, L.; Lee, L.; Tremblay, J.-F.; Drolet, P.; Dubé, P. Impact of electrocautery and hyperthermic intraperitoneal chemotherapy on intestinal microvasculature in a murine model. Int. J. Hyperth. 2016, 32, 1–5.
- Tang, L.; Duan, R.; Zhong, Y.-J.; Firestone, R.; Hong, Y.-P.; Li, J.-G.; Xin, Y.-C.; Wu, H.-L.; Li, Y. Synthesis, identification and in vivo studies of tumor-targeting agent peptide doxorubicin (PDOX) to treat peritoneal carcinomatosis of gastric cancer with similar efficacy but reduced toxicity. Mol. Cancer 2014, 13, 44.
- Tang, L.; Mei, L.-J.; Yang, X.-J.; Huang, C.-Q.; Zhou, Y.-F.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: Evidence from an experimental study. J. Transl. Med. 2011, 9, 53.
- Park, E.J.; Ahn, J.; Gwak, S.W.; Park, K.S.; Baik, S.H.; Hwang, S.-J. Pharmacologic Properties of the Carrier Solutions for Hyperthermic Intraperitoneal Chemotherapy: Comparative Analyses between Water and Lipid Carrier Solutions in the Rat Model. Ann. Surg. Oncol. 2018, 25, 3185–3192.
- Raue, W.; Kilian, M.; Braumann, C.; Atanassow, V.; Makareinis, A.; Caldenas, S.; Schwenk, W.; Hartmann, J. Multimodal approach for treatment of peritoneal surface malignancies in a tumour-bearing rat model. Int. J. Color. Dis. 2010, 25, 245–250.
- Bespalov, V.G.; Kireeva, G.S.; Belyaeva, O.A.; Kalinin, O.E.; Senchik, K.Y.; Stukov, A.N.; Gafton, G.I.; Guseynov, K.D.; Belyaev, A.M. Both heat and new chemotherapeutic drug dioxadet in hyperthermic intraperitoneal chemoperfusion improved survival in rat ovarian cancer model. J. Surg. Oncol. 2016, 113, 438–442.
- Aghayeva, A.; Benlice, C.; Bilgin, I.A.; Atukeren, P.; Dogusoy, G.; Demir, F.; Atasoy, D.; Baca, B. The Effects of Hyperthermic Intraperitoneal Chemoperfusion on Colonic Anastomosis: An Experimental Study in a Rat Model. Tumori J. 2017, 103, 307–313.
- Klaver, Y.L.B.; Hendriks, T.; Lomme, R.; Rutten, H.J.T.; Bleichrodt, R.P.; de Hingh, I. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery for peritoneal carcinomatosis in an experimental model. BJS 2010, 97, 1874–1880.
- Derrien, A.; Gouard, S.; Maurel, C.; Gaugler, M.-H.; Bruchertseifer, F.; Morgenstern, A.; Faivre-Chauvet, A.; Classe, J.-M.; Chérel, M. Therapeutic Efficacy of Alpha-RIT Using a 213Bi-Anti-hCD138 Antibody in a Mouse Model of Ovarian Peritoneal Carcinomatosis. Front. Med. 2015, 2.
- Francescutti, V.; Rivera, L.; Seshadri, M.; Kim, M.; Haslinger, M.; Camoriano, M.; Attwood, K.; Kane, J.M.; Skitzki, J.J. The benefit of intraperitoneal chemotherapy for the treatment of colorectal carcinomatosis. Oncol. Rep. 2013, 30, 35–42.
- Qiu, C.; Li, Y.; Liang, X.; Qi, Y.; Chen, Y.; Meng, X.; Zheng, H.; Xu, Y.; Cai, S.; Cai, G.; et al. A study of peritoneal metastatic xenograft model of colorectal cancer in the treatment of hyperthermic intraperitoneal chemotherapy with Raltitrexed. Biomed. Pharmacother. 2017, 92, 149–156.
- Badrudin, D.; Perrault-Mercier, C.; Bouchard-Fortier, A.; Hubert, J.; Leblond, F.A.; Sideris, L.; Dubé, P. Pharmacokinetics and the effect of heat on intraperitoneal pemetrexed using a murine model. Surg. Oncol. 2016, 25, 435–440.
- Piché, N.; Leblond, F.A.; Sidéris, L.; Pichette, V.; Drolet, P.; Fortier, L.-P.; Mitchell, A.; Dubé, P. Rationale for Heating Oxaliplatin for the Intraperitoneal Treatment of Peritoneal Carcinomatosis. Ann. Surg. 2011, 254, 138–144.
- Gesson-Paute, A.; Ferron, G.; Thomas, F.; de Lara, E.C.; Chatelut, E.; Querleu, D. Pharmacokinetics of Oxaliplatin During Open Versus Laparoscopically Assisted Heated Intraoperative Intraperitoneal Chemotherapy (HIPEC): An Experimental Study. Ann. Surg. Oncol. 2007, 15, 339–344.
- Lemoine, L.; Thijssen, E.; Carleer, R.; Cops, J.; Lemmens, V.; Van Eyken, P.; Sugarbaker, P.; van der Speeten, K. Body surface area-based versus concentration-based intraperitoneal perioperative chemotherapy in a rat model of colorectal peritoneal surface malignancy: Pharmacologic guidance towards standardization. Oncotarget 2019, 10, 1407–1424.
- Liesenfeld, L.F.; Hillebrecht, H.C.; Klose, J.; Schmidt, T.; Schneider, M. Impact of Perfusate Concentration on Hyperthermic Intraperitoneal Chemotherapy Efficacy and Toxicity in a Rodent Model. J. Surg. Res. 2020, 253, 262–271.
- McCabe-Lankford, E.; Peterson, M.; McCarthy, B.; Brown, A.J.; Terry, B.; Galarza-Paez, L.; Levi-Polyachenko, N. Murine Models of Intraperitoneal Perfusion for Disseminated Colorectal Cancer. J. Surg. Res. 2019, 233, 310–322.
- Badrudin, D.; Sideris, L.; Perrault-Mercier, C.; Hubert, J.; Leblond, F.A.; Dubé, P. Comparison of open and closed abdomen techniques for the delivery of intraperitoneal pemetrexed using a murine model. J. Surg. Oncol. 2018, 117, 1318–1322.
- Sánchez-García, S.; Padilla-Valverde, D.; Villarejo-Campos, P.; Martín-Fernández, J.; García-Rojo, M.; Rodríguez-Martínez, M. Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat CO2 recirculation system. Surgery 2014, 155, 719–725.
- Padilla-Valverde, D.; Villarejo, P.; Redondo, J.; Oyarzabal, J.; Estella, A.; Palomino, T.; Fernandez, E.; Sanchez, S.; Faba, P.; Baladron, V.; et al. Laparoscopic cytoreductive surgery and HIPEC is effective regarding peritoneum tissue paclitaxel distribution. Clin. Transl. Oncol. 2019, 21, 1260–1269.
- Ortega-Deballon, P.; Facy, O.; Jambet, S.; Magnin, G.; Cotte, E.; Beltramo, J.L.; Chauffert, B.; Rat, P. Which Method to Deliver Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin? An Experimental Comparison of Open and Closed Techniques. Ann. Surg. Oncol. 2010, 17, 1957–1963.
- Ortega-Deballon, P.; Facy, O.; Magnin, G.; Piard, F.; Chauffert, B.; Rat, P. Using a heating cable within the abdomen to make hyperthermic intraperitoneal chemotherapy easier: Feasibility and safety study in a pig model. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 324–328.
- Oršolic, N.; Car, N.; Lisicic, D.; Benkovic, V.; Knežević, A.H.; Domagoj, D.; Petrik, J. Synergism between Propolis and Hyperthermal Intraperitoneal Chemotherapy with Cisplatin on Ehrlich Ascites Tumor in Mice. J. Pharm. Sci. 2013, 102, 4395–4405.
- Carlier, C.; Laforce, B.; van Malderen, S.J.; Gremonprez, F.; Tucoulou, R.; Villanova, J.; de Wever, O.; Vincze, L.; Vanhaecke, F.; Ceelen, W. Nanoscopic tumor tissue distribution of platinum after intraperitoneal administration in a xenograft model of ovarian cancer. J. Pharm. Biomed. Anal. 2016, 131, 256–262.
- Miailhe, G.; Arfi, A.; Mirshahi, M.; Eveno, C.; Pocard, M.; Touboul, C. A new animal model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing mice in the treatment of peritoneal carcinomatosis of ovarian origin. J. Visc. Surg. 2018, 155, 183–189.
- Kudo, M.; Asao, T.; Hashimoto, S.; Kuwano, H. Closed continuous hyperthermic peritoneal perfusion model in mice with peritoneal dissemination of colon 26. Int. J. Hyperth. 2004, 20, 441–450.
- Bespalov, V.G.; Kireeva, G.S.; Belyaeva, O.A.; Senchik, K.Y.; Stukov, A.N.; Maydin, M.A.; Semenov, A.L.; Gafton, G.I.; Guseynov, K.D.; Belyaev, A.M. Experimental study of antitumour activity and effects on leukocyte count of intraperitoneal administration and hyperthermic intraperitoneal chemoperfusion (HIPEC) with dioxadet in a rat model of ovarian cancer. J. Chemother. 2016, 28, 203–209.
- Aarts, F.; Bleichrodt, R.P.; de Man, B.; Lomme, R.; Boerman, O.C.; Hendriks, T. The Effects of Adjuvant Experimental Radioimmunotherapy and Hyperthermic Intraperitoneal Chemotherapy on Intestinal and Abdominal Healing after Cytoreductive Surgery for Peritoneal Carcinomatosis in the Rat. Ann. Surg. Oncol. 2008, 15, 3299–3307.
- Bouquet, W.; Deleye, S.; Staelens, S.; de Smet, L.; Van Damme, N.; DeBergh, I.; Ceelen, W.P.; de Vos, F.; Remon, J.P.; Vervaet, C. Antitumour Efficacy of Two Paclitaxel Formulations for Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in an In Vivo Rat Model. Pharm. Res. 2011, 28, 1653–1660.
- Pelz, J.O.W.; Doerfer, J.; Hohenberger, W.; Meyer, T. A new survival model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing rats in the treatment of peritoneal carcinomatosis. BMC Cancer 2005, 5, 56.
- Klaver, Y.L.B.; Hendriks, T.; Lomme, R.; Rutten, H.J.T.; Bleichrodt, R.P.; de Hingh, I. Intraoperative versus Early Postoperative Intraperitoneal Chemotherapy after Cytoreduction for Colorectal Peritoneal Carcinomatosis: An Experimental Study. Ann. Surg. Oncol. 2011, 19, 475–482.
- Klaver, Y.L.B.; Hendriks, T.; Lomme, R.; Rutten, H.J.T.; Bleichrodt, R.P.; de Hingh, I. Hyperthermia and Intraperitoneal Chemotherapy for the Treatment of Peritoneal Carcinomatosis. Ann. Surg. 2011, 254, 125–130.
- Colin, P.; de Smet, L.; Vervaet, C.; Remon, J.-P.; Ceelen, W.; Van Bocxlaer, J.; Boussery, K.; Vermeulen, A. A Model Based Analysis of IPEC Dosing of Paclitaxel in Rats. Pharm. Res. 2014, 31, 2876–2886.
- Gremonprez, F.; Willaert, W.; Ceelen, W. Animal models of colorectal peritoneal metastasis. Pleura Peritoneum 2016, 1, 23–43.
- Löke, D.R.; Helderman, R.F.C.P.A.; Rodermond, H.M.; Tanis, P.J.; Streekstra, G.J.; Franken, N.A.P.; Oei, A.L.; Crezee, J.; Kok, H.P. Demonstration of treatment planning software for hyperthermic intraperitoneal chemotherapy in a rat model. Int. J. Hyperth. 2021, 38, 38–54.
- Löke, D.R.; Helderman, R.F.C.P.A.; Sijbrands, J.; Rodermond, H.M.; Tanis, P.J.; Franken, N.A.P.; Oei, A.L.; Kok, H.P.; Crezee, J. A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers 2020, 12, 3516.




