1000/1000
Hot
Most Recent

Fructose is a main dietary sugar involved in the excess sugar intake-mediated progression of cardiovascular diseases and cardiac arrhythmias. Chronic intake of fructose has been the focus on the possible contributor to the metabolic diseases and cardiac inflammation. Recently, the small intestine was identified to be a major organ in fructose metabolism. The overconsumption of fructose induces dysbiosis of the gut microbiota, which, in turn, increases intestinal permeability and activates host inflammation. Endotoxins and metabolites of the gut microbiota, such as lipopolysaccharide, trimethylamine N-oxide, and short-chain fatty acids, also influence the host inflammation and cardiac biofunctions. Thus, high-fructose diets cause heart–gut axis disorders that promote cardiac arrhythmia. Understanding how gut microbiota dysbiosis-mediated inflammation influences the pathogenesis of cardiac arrhythmia may provide mechanisms for cardiac arrhythmogenesis.
| Probiotics | Protocol | Outcomes | References |
|---|---|---|---|
| L. rhamnosus GR-1 | Coronary artery ligation rats fed rGR-1 (109 CFU/g, daily) in drinking water for 6 weeks. | Reduced cardiac hypertrophy and LV dysfunction. | [28] |
| L. acidophilus, Bifidobacterium bifidum, L. reuteri, L. fermentum | Patients with diabetic and coronary heart disease received vitamin D (50,000 IU) plus probiotics (8 × 109 CFU, every 2 weeks) for 12 weeks. | Reduced inflammation and increased antioxidant capacity, nitric oxide, glycemic control, and high-density lipoprotein. | [29] |
| B. breve, L. casei, L. bulgaricus L. acidophilus |
Rats fed probiotics (2 × 106 CFU/mL, daily) for 2 weeks in response to isoproterenol-induced myocardial injury. | Reduced oxidative stress and inflammation and increased cardiac function. | [30] |
| L. curvatus HY7601, L. plantarum KY1032 | Rats fed a high-fructose diet (70% w/w) for 3 weeks followed by a probiotic (109–1010 CFU, daily) for 3 weeks. | Reduced oxidative stress, insulin resistance, and levels of plasma glucose and triglycerides. | [31] |
| L. rhamnosus LS-8, L. crustorum MN047 | Mice fed a high-fructose high fact diet (45% kcal fat, 10% w/v fructose) and a probiotic (109 CFU, daily) for 10 weeks. | Reduced insulin resistance and inflammation. | [32] |
| L. kefiri | Mice fed fructose (20% w/v) and a probiotic (108 CFU, every 2 days) for 6 weeks. | Reduced adipose tissue expansion, plasma triglyceride and leptin levels, and inflammation. | [33] |
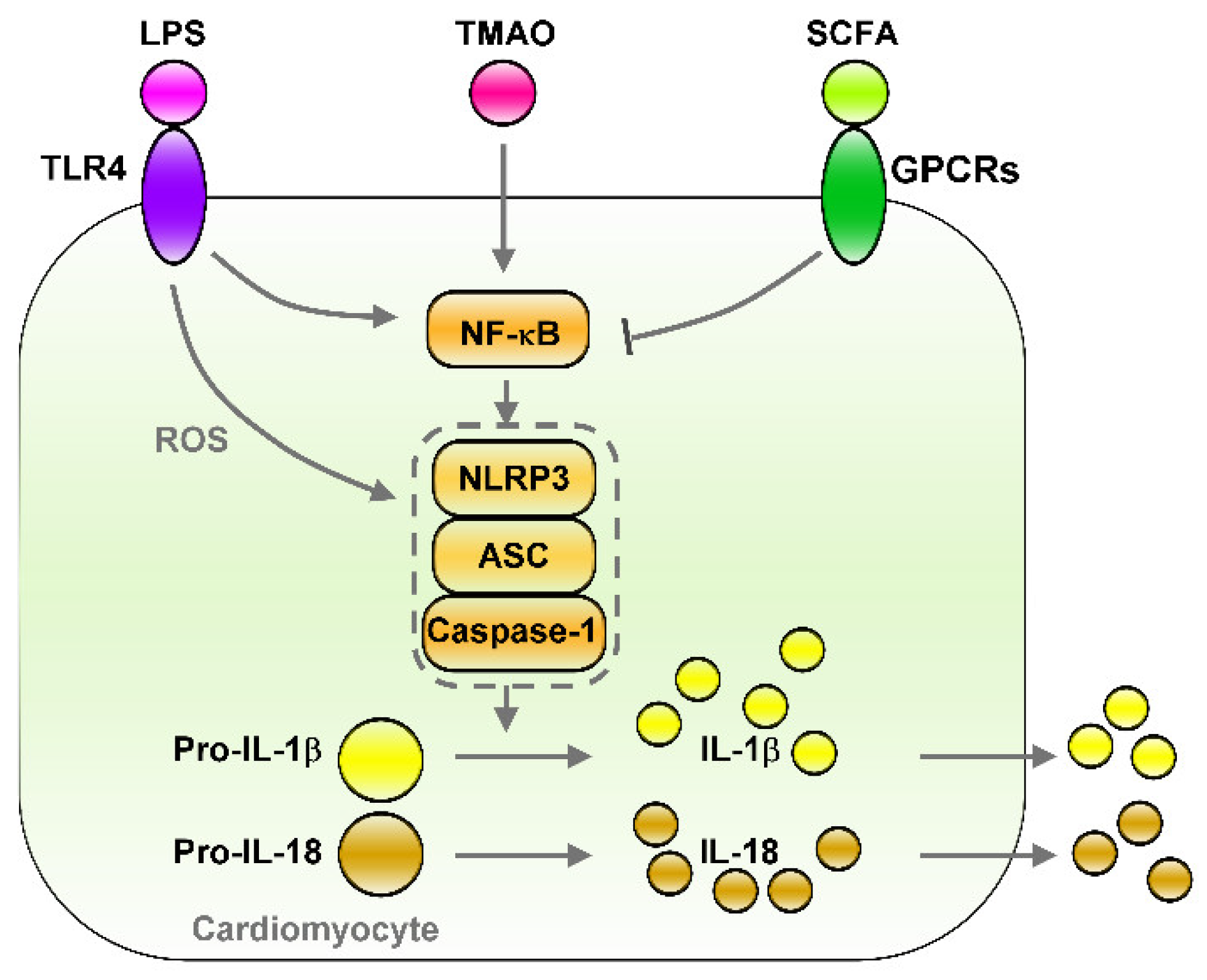 Figure 1. Effects of gut microbiota-derived endotoxin and metabolites on the regulation of NF-κB/NLRP3 inflammasome signaling. Gut microbiota-derived endotoxin or metabolite signaling (such as LPS/TLR4, TMAO, and SCFA/GPCRs) that altered down-stream NF-κB/NLRP3 inflammasome signaling and their effects on cardiac physiology. LPS/TLR4 and TMAO activates NF-κB/NLRP3 axis and induces secretion of IL-1β/IL-18. However, SCFA/GPCRs signaling inhibit NF-κB/NLRP3 signaling. LPS: lipopolysaccharide, TLR4: toll-like receptor 4, TMAO: trimethylamine-N-oxide, SCFA: short-chain fatty acid, GPCRs: G-protein coupled receptors, ROS: Reactive oxygen species, NLRP3: NLR family pyrin domain containing 3, ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain, Pro-IL-1β: Pro-form interleukin 1 beta, Pro-IL-18: pro form interleukin 18, IL-1β: interleukin 1 beta, IL-18: interleukin 18.
Figure 1. Effects of gut microbiota-derived endotoxin and metabolites on the regulation of NF-κB/NLRP3 inflammasome signaling. Gut microbiota-derived endotoxin or metabolite signaling (such as LPS/TLR4, TMAO, and SCFA/GPCRs) that altered down-stream NF-κB/NLRP3 inflammasome signaling and their effects on cardiac physiology. LPS/TLR4 and TMAO activates NF-κB/NLRP3 axis and induces secretion of IL-1β/IL-18. However, SCFA/GPCRs signaling inhibit NF-κB/NLRP3 signaling. LPS: lipopolysaccharide, TLR4: toll-like receptor 4, TMAO: trimethylamine-N-oxide, SCFA: short-chain fatty acid, GPCRs: G-protein coupled receptors, ROS: Reactive oxygen species, NLRP3: NLR family pyrin domain containing 3, ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain, Pro-IL-1β: Pro-form interleukin 1 beta, Pro-IL-18: pro form interleukin 18, IL-1β: interleukin 1 beta, IL-18: interleukin 18.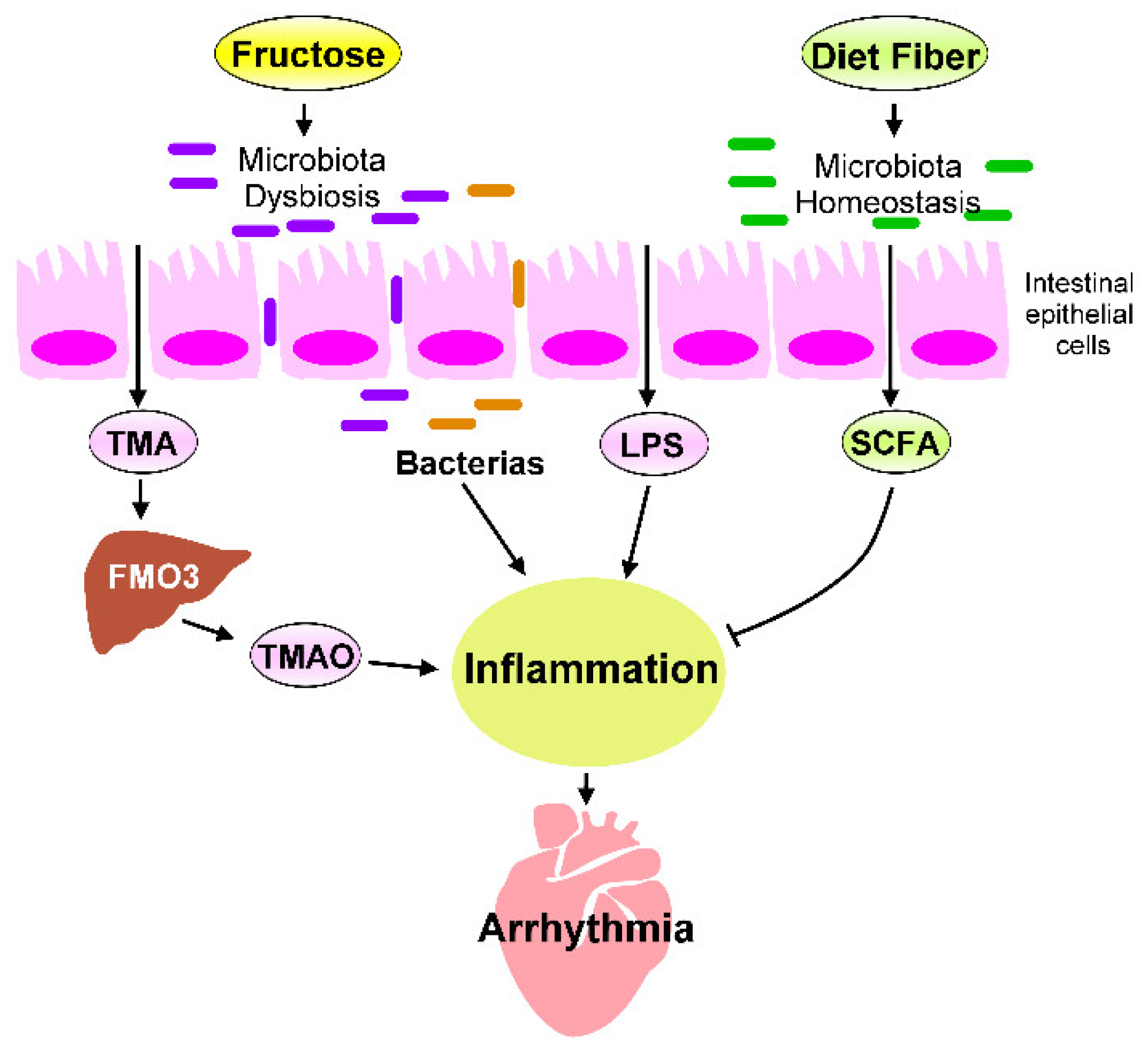 Figure 2. Fructose-mediated heart–gut axis disorder that promotes inflammation and cardiac arrhythmogenesis. Dietary components, such as fructose or dietary fiber, serve as crucial environmental factors that influence the homeostasis of gut microbiota and alter gut microbiota-derived metabolites. Excessive fructose intake promotes microbiota dysbiosis, which increases the production of trimethylamine (TMA), which is then converted into trimethylamine-N-oxide (TMAO) by the flavin-containing monooxygenase 3 (FMO3) expressed in the liver. SCFAs are generated through the fermentation of dietary fibers by gut microbiota. SCFAs are crucial players in regulating the beneficial effect of dietary fibers. The microbiota endotoxin and metabolites, such as lipopolysaccharide (LPS), TMAO, and SCFAs, mechanistically regulate the chronic inflammation that affects cardiac rhythm. Targeting inflammation caused by imbalanced intestinal flora may prevent cardiac arrhythmogenesis.
Figure 2. Fructose-mediated heart–gut axis disorder that promotes inflammation and cardiac arrhythmogenesis. Dietary components, such as fructose or dietary fiber, serve as crucial environmental factors that influence the homeostasis of gut microbiota and alter gut microbiota-derived metabolites. Excessive fructose intake promotes microbiota dysbiosis, which increases the production of trimethylamine (TMA), which is then converted into trimethylamine-N-oxide (TMAO) by the flavin-containing monooxygenase 3 (FMO3) expressed in the liver. SCFAs are generated through the fermentation of dietary fibers by gut microbiota. SCFAs are crucial players in regulating the beneficial effect of dietary fibers. The microbiota endotoxin and metabolites, such as lipopolysaccharide (LPS), TMAO, and SCFAs, mechanistically regulate the chronic inflammation that affects cardiac rhythm. Targeting inflammation caused by imbalanced intestinal flora may prevent cardiac arrhythmogenesis.