Fructose is a main dietary sugar involved in the excess sugar intake-mediated progression of cardiovascular diseases and cardiac arrhythmias. Chronic intake of fructose has been the focus on the possible contributor to the metabolic diseases and cardiac inflammation. Recently, the small intestine was identified to be a major organ in fructose metabolism. The overconsumption of fructose induces dysbiosis of the gut microbiota, which, in turn, increases intestinal permeability and activates host inflammation. Endotoxins and metabolites of the gut microbiota, such as lipopolysaccharide, trimethylamine N-oxide, and short-chain fatty acids, also influence the host inflammation and cardiac biofunctions. Thus, high-fructose diets cause heart–gut axis disorders that promote cardiac arrhythmia. Understanding how gut microbiota dysbiosis-mediated inflammation influences the pathogenesis of cardiac arrhythmia may provide mechanisms for cardiac arrhythmogenesis.
- arrhythmia
- fructose
- heart–gut axis
- inflammation
- microbiota
1. Introduction
2. Therapeutic Strategies for Fructose-Mediated Inflammation
The targeting of inflammation can potentially reduce the risk of cardiac arrhythmia, and the blocking of gut microbiota-mediated inflammation by reducing fructose intake, inhibiting inflammation signaling, and administering probiotics and dietary short-chain fatty acids (SCFAs) may reduce the risk of CVDs. These approaches can ground novel therapies that target inflammation-associated cardiac arrhythmias and atrial fibrillation (AF).2.1. Dietary Interventions
2.2. Probiotics for Controlling Cardiac Inflammation
| Probiotics | Protocol | Outcomes | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. rhamnosus | GR-1 | Coronary artery ligation rats fed rGR-1 (10 | 9 | CFU/g, daily) in drinking water for 6 weeks. | Reduced cardiac hypertrophy and LV dysfunction. | [28] | |||||||
| L. acidophilus | , | Bifidobacterium bifidum | , | L. reuteri | , | L. fermentum | Patients with diabetic and coronary heart disease received vitamin D (50,000 IU) plus probiotics (8 × 10 | 9 | CFU, every 2 weeks) for 12 weeks. | Reduced inflammation and increased antioxidant capacity, nitric oxide, glycemic control, and high-density lipoprotein. | [29] | ||
| B. breve | , | L. casei | , | L. bulgaricus | L. acidophilus | Rats fed probiotics (2 × 10 | 6 | CFU/mL, daily) for 2 weeks in response to isoproterenol-induced myocardial injury. | Reduced oxidative stress and inflammation and increased cardiac function. | [30] | |||
| L. curvatus HY7601 | , | L. plantarum KY1032 | Rats fed a high-fructose diet (70% | w | / | w | ) for 3 weeks followed by a probiotic (10 | 9 | –10 | 10 | CFU, daily) for 3 weeks. | Reduced oxidative stress, insulin resistance, and levels of plasma glucose and triglycerides. | [31] |
| L. rhamnosus LS-8 | , | L. crustorum MN047 | Mice fed a high-fructose high fact diet (45% kcal fat, 10% | w | / | v | fructose) and a probiotic (10 | 9 | CFU, daily) for 10 weeks. | Reduced insulin resistance and inflammation. | [32] | ||
| L. kefiri | Mice fed fructose (20% | w | / | v | ) and a probiotic (10 | 8 | CFU, every 2 days) for 6 weeks. | Reduced adipose tissue expansion, plasma triglyceride and leptin levels, and inflammation. | [33] |
2.3. Effects of SCFAs on Controlling Inflammation
2.3. Effects of SCFAs on Controlling Inflammation
SCFAs are the ligands for G protein-coupled receptors (GPCRs), which contain GPR43, GPR41, and GPR109A, that trigger anti-inflammatory signaling cascades [34]. SCFAs also modulate immune responses, partially by affecting gene expressions and the epigenome through the inhibition of histone deacetylases (HDAC) [35][36]. SCFAs are saturated aliphatic organic acids that comprise one to six carbon atoms, of which propionate, acetate, and butyrate are the most abundant and are produced by anaerobic fermentation of dietary fiber in the gut [37]. Firmicutes (gram-positive) and Bacteroidetes (gram-negative) are the most abundant phyla in the intestines, with members of Firmicutes mainly producing butyrate, whereas acetate and propionate are the primary metabolic end products of members of Bacteroidetes [38]. SCFA butyrate protects intestinal epithelial cells and stabilizes hypoxia-induced factors and, thus, attenuates local and systemic inflammation [39]. Dietary-derived butyrate inhibits innate lymphoid cells and subsequently reduces lung inflammation, airway hyperreactivity, and eosinophilia in an allergic asthma murine model [40]. SCFAs can reduce impairments of the intestinal epithelial barrier due to their protection against high-fructose-diet-induced neuroinflammation [41]. Clinical studies have revealed that daily oral supplementation of 1010 of Akkermansia muciniphila bacteria (live or pasteurized) can improve insulin sensitivity and reduce insulinemia and plasma total cholesterol in overweight or obese insulin-resistant volunteers relative to a placebo. After 3 months of supplementation, Akkermansia muciniphila reduced the levels of the relevant blood markers for liver dysfunction and inflammation [42]. The butyrate–GPR109A axis inhibited LPS-induced NF-κB activation in colonic cell lines and in the colon of mice [43]. SCFAs, as an HDAC inhibitor, can protect the intestinal barrier from disruption by inhibiting the LPS–NLRP3 inflammasome axis [44]. Acetate diminishes NLRP3 inflammasome activation through GPR43 and Ca2+-dependent mechanisms, which underscores the mechanism of metabolite-attenuated NLRP3 inflammasome activity that mitigates CVD development [45]. As illustrated in Figure 12, SCFA treatment can inhibit NF-κB/NLRP3 signaling, which may prevent inflammation-associated heart arrhythmia.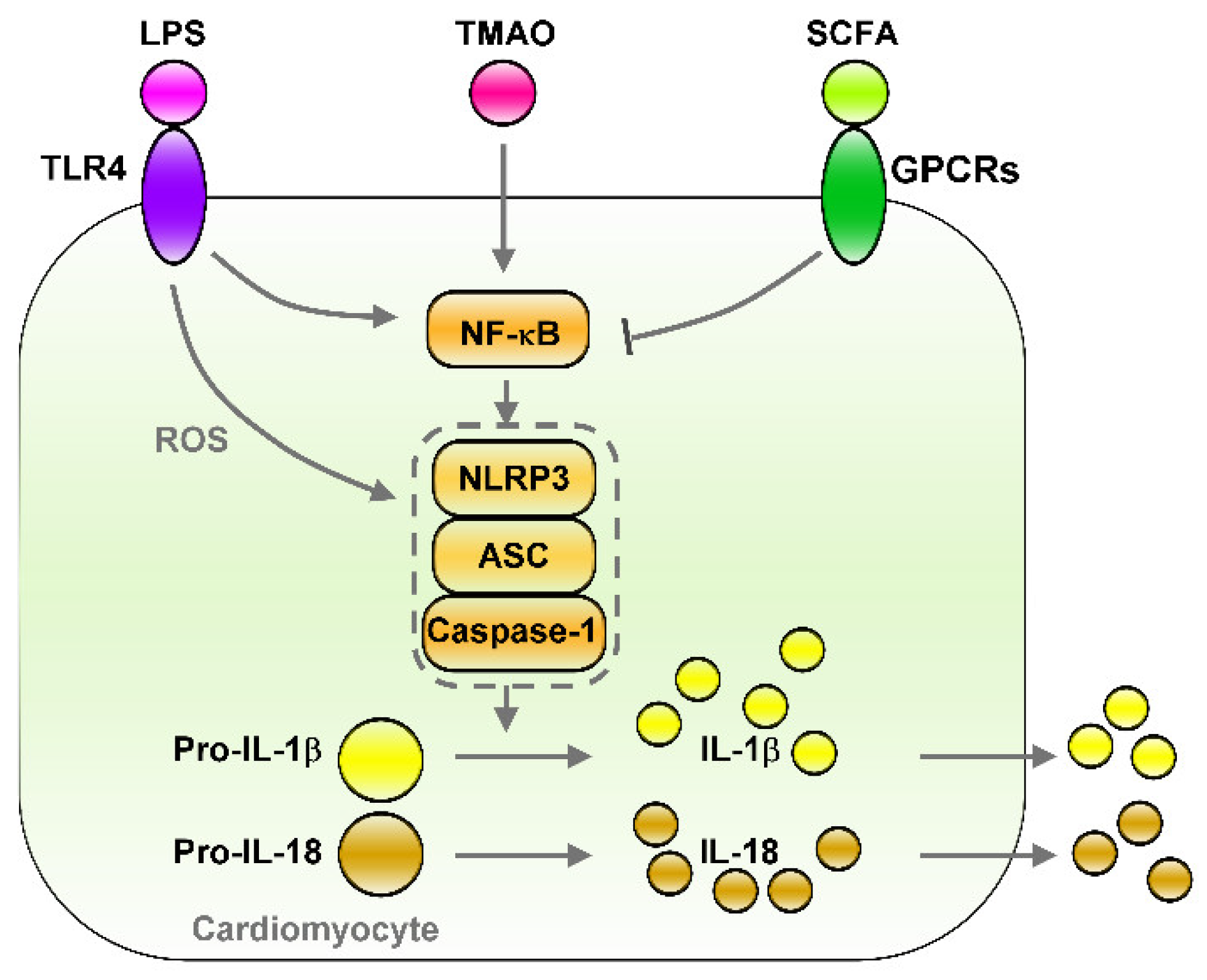 Figure 12. Effects of gut microbiota-derived endotoxin and metabolites on the regulation of NF-κB/NLRP3 inflammasome signaling. Gut microbiota-derived endotoxin or metabolite signaling (such as LPS/TLR4, TMAO, and SCFA/GPCRs) that altered down-stream NF-κB/NLRP3 inflammasome signaling and their effects on cardiac physiology. LPS/TLR4 and TMAO activates NF-κB/NLRP3 axis and induces secretion of IL-1β/IL-18. However, SCFA/GPCRs signaling inhibit NF-κB/NLRP3 signaling. LPS: lipopolysaccharide, TLR4: toll-like receptor 4, TMAO: trimethylamine-N-oxide, SCFA: short-chain fatty acid, GPCRs: G-protein coupled receptors, ROS: Reactive oxygen species, NLRP3: NLR family pyrin domain containing 3, ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain, Pro-IL-1β: Pro-form interleukin 1 beta, Pro-IL-18: pro form interleukin 18, IL-1β: interleukin 1 beta, IL-18: interleukin 18.
Figure 12. Effects of gut microbiota-derived endotoxin and metabolites on the regulation of NF-κB/NLRP3 inflammasome signaling. Gut microbiota-derived endotoxin or metabolite signaling (such as LPS/TLR4, TMAO, and SCFA/GPCRs) that altered down-stream NF-κB/NLRP3 inflammasome signaling and their effects on cardiac physiology. LPS/TLR4 and TMAO activates NF-κB/NLRP3 axis and induces secretion of IL-1β/IL-18. However, SCFA/GPCRs signaling inhibit NF-κB/NLRP3 signaling. LPS: lipopolysaccharide, TLR4: toll-like receptor 4, TMAO: trimethylamine-N-oxide, SCFA: short-chain fatty acid, GPCRs: G-protein coupled receptors, ROS: Reactive oxygen species, NLRP3: NLR family pyrin domain containing 3, ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain, Pro-IL-1β: Pro-form interleukin 1 beta, Pro-IL-18: pro form interleukin 18, IL-1β: interleukin 1 beta, IL-18: interleukin 18.
2.4. HDACs’ Inhibition of Cardiac Inflammation
3. Conclusions and Future Perspectives
The heart–gut axis is a potential target for cardiovascular therapy. High-fructose diets can induce inflammation and metabolic disorders in the heart–gut axis due to cardiac arrhythmogenesis (Figure 23). Excessive fructose intake causes dysbiosis of the microbiota, which leads to increased gut barrier permeability, inflammation, progression of metabolic disease, and IR. Reducing sugar consumption and consuming dietary fiber, a Med-diet, probiotics, and SCFAs and receiving HDACi treatment can decrease high-fructose-diet-induced chronic inflammation. The targeting of excessive fructose intake-associated inflammation in the heart–gut axis can prevent cardiac arrhythmia. However, in general, studies have not determined: (1) the specific microbiota strains that can inhibit excessive fructose intake–induced heart disease; (2) how to maintain a healthy diet that avoids TMAO accumulation in the human body and how to prevent TMAO-mediated CVDs; or (3) how the SCFAs and HDACi regulate signaling that may exert an anti-inflammatory activity in the heart. Future investigations should focus on how one can maintain a balanced diet to keep their gut microbiota homeostasis and avoid systematic inflammation. Microbial population and microbiota biofunction analysis can help researchers explore the effects of probiotics on the heart–gut axis; such effects can ground promising strategies for maintaining gut microbiome homeostasis in particular and cardiac health in general. Although TMAO is a risk factor for CVD progression, TMAO mechanisms mediated CVDs, and therapeutic strategies to inhibit TMAO signaling should be explored for CVD interventions. Moreover, animal studies and clinical trials are required to analyze whether interventions that target microbiota homeostasis, inhibit TMAO signaling, or activate SCFA regulated pathways can reduce the likelihood of adverse cardiac events and prevent cardiac arrhythmogenesis. Thus, targeting the heart–gut axis may reduce the occurrence or severity of cardiac pathogenesis mediated by excess fructose consumption.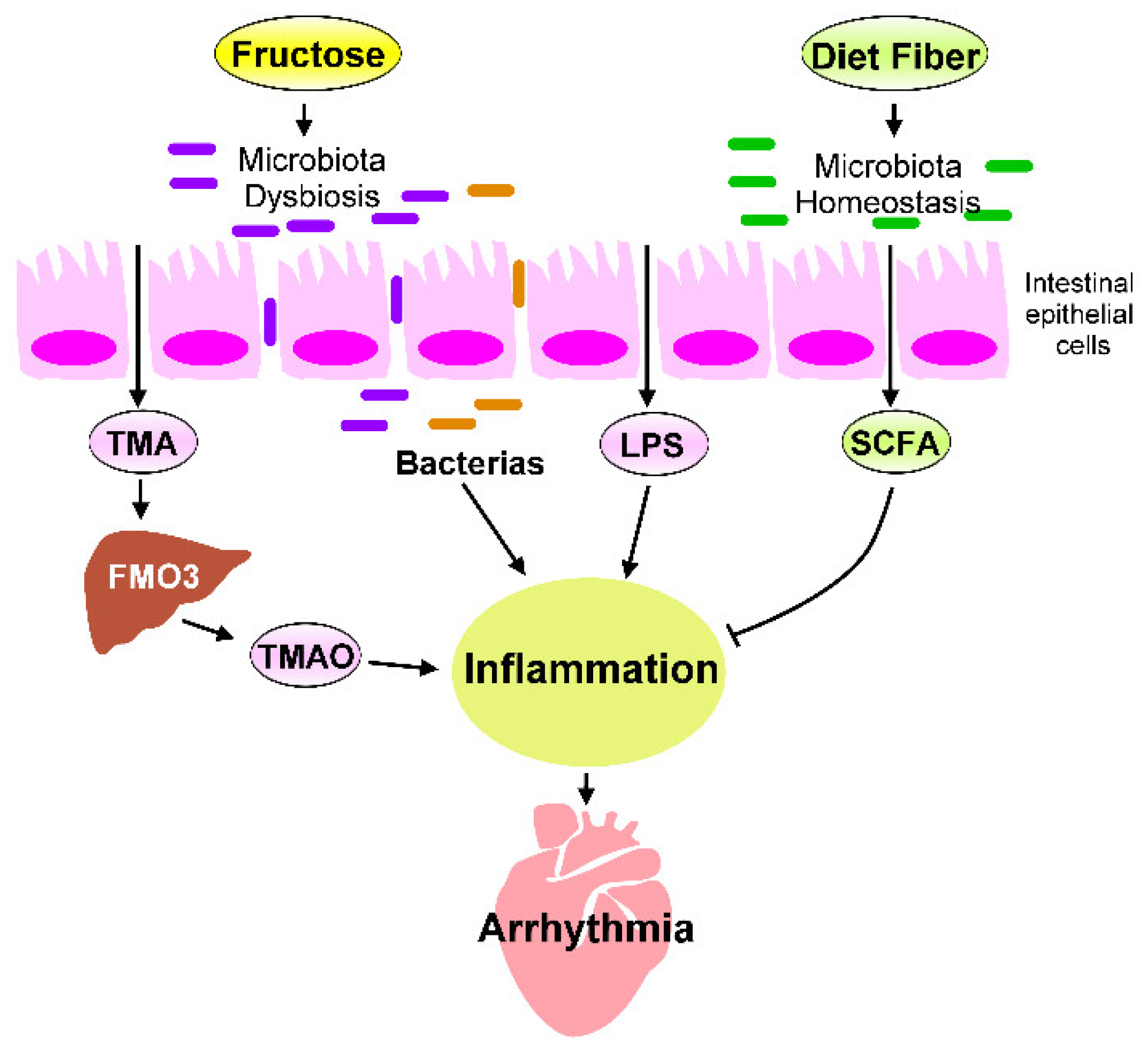 Figure 23. Fructose-mediated heart–gut axis disorder that promotes inflammation and cardiac arrhythmogenesis. Dietary components, such as fructose or dietary fiber, serve as crucial environmental factors that influence the homeostasis of gut microbiota and alter gut microbiota-derived metabolites. Excessive fructose intake promotes microbiota dysbiosis, which increases the production of trimethylamine (TMA), which is then converted into trimethylamine-N-oxide (TMAO) by the flavin-containing monooxygenase 3 (FMO3) expressed in the liver. SCFAs are generated through the fermentation of dietary fibers by gut microbiota. SCFAs are crucial players in regulating the beneficial effect of dietary fibers. The microbiota endotoxin and metabolites, such as lipopolysaccharide (LPS), TMAO, and SCFAs, mechanistically regulate the chronic inflammation that affects cardiac rhythm. Targeting inflammation caused by imbalanced intestinal flora may prevent cardiac arrhythmogenesis.
Figure 23. Fructose-mediated heart–gut axis disorder that promotes inflammation and cardiac arrhythmogenesis. Dietary components, such as fructose or dietary fiber, serve as crucial environmental factors that influence the homeostasis of gut microbiota and alter gut microbiota-derived metabolites. Excessive fructose intake promotes microbiota dysbiosis, which increases the production of trimethylamine (TMA), which is then converted into trimethylamine-N-oxide (TMAO) by the flavin-containing monooxygenase 3 (FMO3) expressed in the liver. SCFAs are generated through the fermentation of dietary fibers by gut microbiota. SCFAs are crucial players in regulating the beneficial effect of dietary fibers. The microbiota endotoxin and metabolites, such as lipopolysaccharide (LPS), TMAO, and SCFAs, mechanistically regulate the chronic inflammation that affects cardiac rhythm. Targeting inflammation caused by imbalanced intestinal flora may prevent cardiac arrhythmogenesis.
References
- Howard, B.V.; Wylie-Rosett, J. Sugar and cardiovascular disease: A statement for healthcare professionals from the Committee on Nutrition of the Council on Nutrition, Physical Activity, and Metabolism of the American Heart Association. Circulation 2002, 106, 523–527.
- Mirtschink, P.; Jang, C.; Arany, Z.; Krek, W. Fructose metabolism, cardiometabolic risk, and the epidemic of coronary artery disease. Eur. Heart J. 2018, 39, 2497–2505.
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J.; American Heart Association Nutrition Committee of the Council on Nutrition; et al. Dietary sugars intake and cardiovascular health: A scientific statement from the American Heart Association. Circulation 2009, 120, 1011–1020.
- Bray, G.A. Fructose and risk of cardiometabolic disease. Curr. Atheroscler. Rep. 2012, 14, 570–578.
- Vartanian, L.R.; Schwartz, M.B.; Brownell, K.D. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am. J. Public Health 2007, 97, 667–675.
- Bray, G.A. Fructose: Pure, white, and deadly? Fructose, by any other name, is a health hazard. J. Diabetes Sci. Technol. 2010, 4, 1003–1007.
- Malik, V.S.; Hu, F.B. Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624.
- Malik, V.S.; Hu, F.B. Sugar-sweetened beverages and cardiometabolic health: An update of the evidence. Nutrients 2019, 11, 8.
- Zhang, D.M.; Jiao, R.Q.; Kong, L.D. High dietary fructose: Direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients 2017, 9, 4.
- Porto, M.L.; Lirio, L.M.; Dias, A.T.; Batista, A.T.; Campagnaro, B.P.; Mill, J.G.; Meyrelles, S.S.; Baldo, M.P. Increased oxidative stress and apoptosis in peripheral blood mononuclear cells of fructose-fed rats. Toxicol. In Vitro 2015, 29, 1977–1981.
- Chan, W.; Smith, B.; Stegall, M.; Borrows, R. Obesity and metabolic syndrome in kidney transplantation: The role of dietary fructose and systemic endotoxemia. Transplantation 2019, 103, 191–201.
- Miller, A.; Adeli, K. Dietary fructose and the metabolic syndrome. Curr. Opin. Gastroenterol. 2008, 24, 204–209.
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157.
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779.
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922.
- Dhingra, R.; Sullivan, L.; Jacques, P.F.; Wang, T.J.; Fox, C.S.; Meigs, J.B.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007, 116, 480–488.
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients 2017, 9, 4.
- Jegatheesan, P.; De Bandt, J.P. Fructose and NAFLD: The multifaceted aspects of fructose metabolism. Nutrients 2017, 9, 230.
- Diamond, D.M.; O’Neill, B.J.; Volek, J.S. Low carbohydrate diet: Are concerns with saturated fat, lipids, and cardiovascular disease risk justified? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 291–300.
- Kelly, T.; Unwin, D.; Finucane, F. Low-carbohydrate diets in the management of obesity and type 2 diabetes: A review from clinicians using the approach in practice. Int. J. Environ. Res. Public Health 2020, 17, 7.
- Unwin, D.J.; Tobin, S.D.; Murray, S.W.; Delon, C.; Brady, A.J. Substantial and sustained improvements in blood pressure, weight and lipid profiles from a carbohydrate restricted diet: An observational study of insulin resistant patients in primary care. Int. J. Environ. Res. Public Health 2019, 16, 15.
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes 2017, 2, e5.
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821.
- Pastori, D.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Tanzilli, G.; Cangemi, R.; Vicario, T.; Catena, M.; Violi, F.; Pignatelli, P. Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxid. Redox Signal. 2015, 23, 682–687.
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344.
- Wang, A.Y.; Sea, M.M.; Ng, K.; Wang, M.; Chan, I.H.; Lam, C.W.-K.; Sanderson, J.; Woo, J. Dietary fiber intake, myocardial injury, and major adverse cardiovascular events among end-stage kidney disease patients: A prospective cohort study. Kidney Int. Rep. 2019, 4, 814–823.
- Hendijani, F.; Akbari, V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 532–541.
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499.
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 50–55.
- Sadeghzadeh, J.; Vakili, A.; Sameni, H.R.; Shadnoush, M.; Bandegi, A.R.; Zahedi Khorasani, M. The effect of oral consumption of probiotics in prevention of heart injury in a rat myocardial infarction model: A histopathological, hemodynamic and biochemical evaluation. Iran. Biomed. J. 2017, 21, 174–181.
- Park, D.Y.; Ahn, Y.T.; Huh, C.S.; McGregor, R.A.; Choi, M.S. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J. Gastroenterol. 2013, 19, 274–283.
- Wang, T.; Yan, H.; Lu, Y.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lu, X. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur. J. Nutr. 2020, 59, 2709–2728.
- Zubiria, M.G.; Gambaro, S.E.; Rey, M.A.; Carasi, P.; Serradell, M.L.A.; Giovambattista, A. Deleterious metabolic effects of high fructose intake: The preventive effect of Lactobacillus kefiri administration. Nutrients 2017, 9, 5.
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 2018, 8, 1091–1115.
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345.
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 2016, 64, 982–992.
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340.
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119.
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Correa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Camara, N.O.S.; de Sales, E.S.E.L.; et al. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019, 27, 750–761 e7.
- Lewis, G.; Wang, B.; Shafiei Jahani, P.; Hurrell, B.P.; Banie, H.; Aleman Muench, G.R.; Maazi, H.; Helou, D.G.; Howard, E.; Galle-Treger, L.; et al. Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front. Immunol. 2019, 10, 2051.
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98.
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103.
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009, 69, 2826–2832.
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. Biochem. 2018, 49, 190–205.
- Xu, M.; Jiang, Z.; Wang, C.; Li, N.; Bo, L.; Zha, Y.; Bian, J.; Zhang, Y.; Deng, X. Acetate attenuates inflammasome activation through GPR43-mediated Ca(2+)-dependent NLRP3 ubiquitination. Exp. Mol. Med. 2019, 51, 83.
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor alpha-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533.
- Lkhagva, B.; Kao, Y.H.; Chen, Y.C.; Chao, T.F.; Chen, S.A.; Chen, Y.J. Targeting histone deacetylases: A novel therapeutic strategy for atrial fibrillation. Eur. J. Pharmacol. 2016, 781, 250–257.
- Liu, F.; Levin, M.D.; Petrenko, N.B.; Lu, M.M.; Wang, T.; Yuan, L.J.; Stout, A.L.; Epstein, J.A.; Patel, V.V. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J. Mol. Cell Cardiol. 2008, 45, 715–723.
- Fan, X.D.; Wan, L.L.; Duan, M.; Lu, S. HDAC11 deletion reduces fructose-induced cardiac dyslipidemia, apoptosis and inflammation by attenuating oxidative stress injury. Biochem. Biophys. Res. Commun. 2018, 503, 444–451.
- McKinsey, T.A. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol. Med. 2011, 17, 434–441.
- Lkhagva, B.; Kao, Y.H.; Lee, T.I.; Lee, T.W.; Cheng, W.L.; Chen, Y.J. Activation of Class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics 2018, 13, 376–385.
- Lkhagva, B.; Chang, S.L.; Chen, Y.C.; Kao, Y.H.; Lin, Y.K.; Chiu, C.T.; Chen, S.A.; Chen, Y.J. Histone deacetylase inhibition reduces pulmonary vein arrhythmogenesis through calcium regulation. Int. J. Cardiol. 2014, 177, 982–989.
- Lee, S.J.; Choi, S.E.; Lee, H.B.; Song, M.W.; Kim, Y.H.; Jeong, J.Y.; Kang, Y.; Kim, H.J.; Kim, T.H.; Jeon, J.Y.; et al. A Class I histone deacetylase inhibitor attenuates insulin resistance and inflammation in palmitate-treated C2C12 myotubes and muscle of HF/HFr diet mice. Front. Pharmacol. 2020, 11, 601448.
