
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Suryadi Ismadji | + 2919 word(s) | 2919 | 2021-06-29 10:39:12 | | | |
| 2 | Peter Tang | Meta information modification | 2919 | 2021-07-09 05:06:49 | | |
Video Upload Options
Cellulose nanofiber (CNF), nanocrystal cellulose (NCC), and bacterial nanocellulose (BC) are the most common nanocellulose used as nanocarriers in drug delivery systems. Modification and functionalization using various processes and chemicals have been carried out to increase the adsorption and drug delivery performance of nanocellulose.
1. Introduction
2. Conversion of Cellulose into Nanocellulose and Its Characteristic
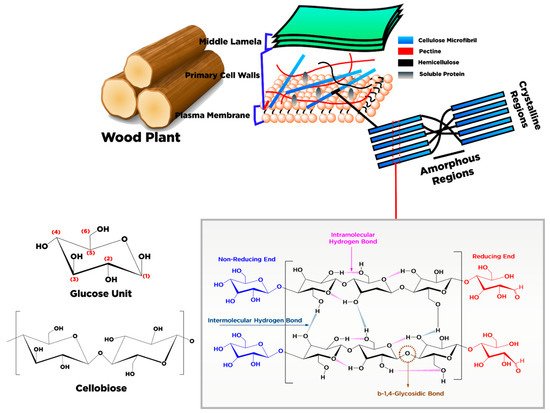
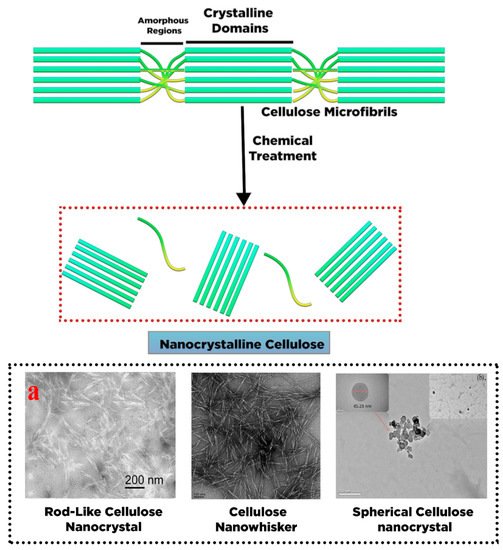
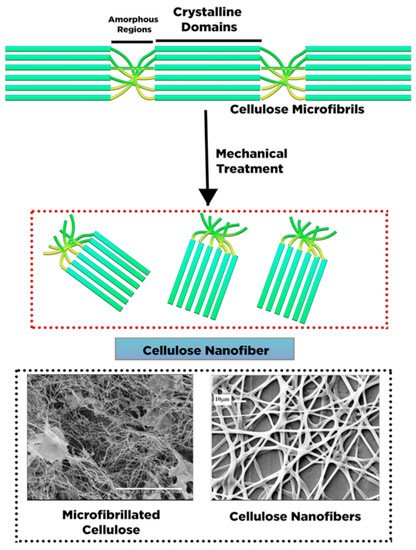
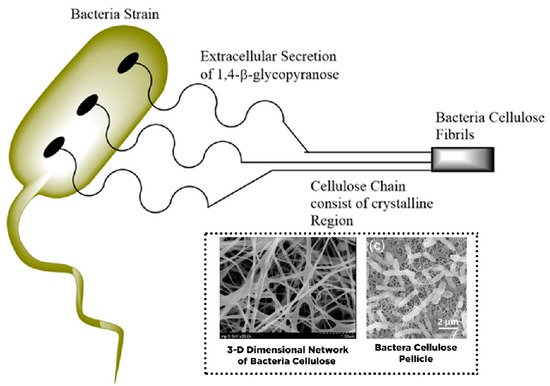
|
Types |
Nanocrystalline Cellulose (NCC) |
Cellulose Nanofibers (CNF) |
Bacterial Cellulose |
|
|---|---|---|---|---|
|
Parameter |
||||
|
Common names |
Cellulose whisker, cellulose nanowhisker, cellulose nanowire, and cellulose nanorod or spherical cellulose nanocrystals |
Cellulose nanofibril, micro fibrillated cellulose, Nanofibrillar cellulose, Nanofibrillated cellulose, and cellulose microfibril |
Microbial cellulose (MC), bacterial nanocellulose (BC), and bio-cellulose (BC) |
|
|
Morphological structure |
Needles like shape, elongated rod-like shape, and spindle shape |
Smooth, extended, and flexible chain |
Twisted ribbons like shape |
|
|
Structure of Nanocellulose |
Crystalline domains |
amorphous and crystalline domains |
Crystalline domains |
|
|
Chain Length |
≥500 |
500–15,000 |
4000–10,000 |
|
|
Crystallinity (%) |
54–88 |
- |
84–88 |
|
|
Other Impurities and contaminant |
Possible to contain hemicellulose, lignin, and pectin |
Possible to contain hemicellulose, lignin, and pectin |
Contain no hemicellulose, lignin, and pectin |
|
|
Size (Length and Diameter) |
Diameter: 5–30 nm and Length: 100–500 nm |
Diameter: 1–100 nm and Length: 500–2000 nm |
Diameter 20–100 nm and several micrometric lengths |
|
|
Process System |
Top-down system |
Top-down system |
Bottom-up system |
|
|
Tensile strength (Gpa) |
7.5–7.7 [23] |
13 |
0.2–0.3 |
|
|
Modulus Young (Gpa) |
110–220 [35] |
Approximately 15 |
18–20 [51] |
|
|
Density (gr/cm3) |
1.6 [53] |
1.42 |
1.1 |
|
|
Characteristics |
Homogenous nanorod form, exceptional aspect ratio (length to diameter), appreciable specific surface area (SSA), biocompatibility, liquid crystalline attribute, inferior breaking expansion, high young’s modulus, hydrophilicity, outstanding mechanical stiffness, tunable surface characteristic due to the reactive hydroxyl group and low density |
Extended length with excellent aspect proportion (length to diameter), superlative surface area, hydrophilicity, biocompatibility and adjustable characteristic through surface modification afforded by high extensive of hydroxyl groups in CNF. |
High crystallinity of nanocellulose (84–88%) and polymerization grade, high water uptake capacity (exceeding 100 times of its weight), remarkable surface area (high aspect proportion of fiber), outstanding tensile strength (young modulus 15–18 Gpa), and flexibility, foldability, moldability, mechanical stability, highly biocompatible material, non-cytotoxic, un-genotoxic and high porosity |
|
3. Surface Chemistry of Nanocellulose for Drug Delivery
References
- Jain, K.K. An overview of drug delivery systems. In Drug Delivery Systems; Springer: New York, NY, USA, 2020.
- Sunasee, R.; Hemraz, U.D.; Ckless, K. Cellulose nanocrystals: A versatile nanoplatform for emerging biomedical applications. Expert Opin. Drug Deliv. 2016, 13, 1243–1256.
- Bamrungsap, S.; Zhao, Z.; Chen, T.; Wand, L.; Li, C.; Fu, T.; Tan, W. Nanotechnology in therapeutics: A focus on nanoparticles as a drug delivery system. Nanomedicine 2012, 7, 1253–1271.
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501.
- Cavallaro, G.; Micciulla, S.; Chiappisi, L.; Lazzara, G. Chitosan-based smart hybrid materials: A physico-chemical perspective. J. Mater. Chem. B 2021, 9, 594–611.
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502.
- Ahmad, A.; Mubarak, N.; Jannat, F.T.; Ashfaq, T.; Santulli, C.; Rizwan, M.; Najda, A.; Bin-Jumah, M.; Abdel-Daim, M.M.; Hussain, S. A Critical Review on the Synthesis of Natural Sodium Alginate Based Composite Materials: An Innovative Biological Polymer for Biomedical Delivery Applications. Processes 2021, 9, 137.
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 dual-targeted multifunctional hyaluronic acid nanogels boost protein delivery to ovarian and breast cancers in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147.
- Qi, X.; Wei, W.; Shen, J.; Dong, W. Salecan polysaccharide-based hydrogels and their applications: A review. J. Mater. Chem. B 2019, 7, 2577–2587.
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides. A review. Carbohydr. Polym. 2019, 225, 115210.
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719.
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781.
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500.
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325.
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp Pap. Res. J. 2014, 29, 105–118.
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent advances in bacterial cellulose. Cellulose 2014, 21, 1–30.
- Foo, M.L.; Tan, C.R.; Lim, P.D.; Ooi, C.W.; Tan, K.W.; Chew, I.M.L. Surface-modified nanocrystalline cellulose from oil palm empty fruit bunch for effective binding of curcumin. Int. J. Biol. Macromol. 2019, 138, 1064–1071.
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.-H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376.
- Dufresne, A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr. Opin. Colloid Interface Sci. 2017, 29, 1–8.
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108.
- Wertz, J.-L.; Bedue, O.; Mercier, J.P. Cellulose Science and Technology; EPFL Press: Lausanne, Switzerland, 2010.
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Medronho, B.; Lindman, B. Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv. Colloid Interface Sci. 2015, 222, 502–508.
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590.
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262.
- Li, N.; Lu, W.; Yu, J.; Xiao, Y.; Liu, S.; Gan, L.; Huang, J. Rod-like cellulose nanocrystal/cis-aconityl-doxorubicin prodrug: A fluorescence-visible drug delivery system with enhanced cellular uptake and intracellular drug controlled release. Mater. Sci. Eng. C 2018, 91, 179–189.
- Jia, B.; Li, Y.; Yang, B.; Xiao, D.; Zhang, S.; Rajulu, A.V.; Kondo, T.; Zhang, L.; Zhou, J. Effect of microcrystal cellulose and cellulose whisker on biocompatibility of cellulose-based electrospun scaffolds. Cellulose 2013, 20, 1911–1923.
- Dash, R.; Ragauskas, A.J. Synthesis of a novel cellulose nanowhisker-based drug delivery system. RSC Adv. 2012, 2, 3403–3409.
- Biswas, S.K.; Sano, H.; Yang, X.; Tanphichai, S.; Shams, M.I.; Yano, H. Highly thermal-resilient AgNW transparent electrode and optical device on thermomechanically superstable cellulose nanorod-reinforced nanocomposites. Adv. Opt. Mater. 2019, 7, 1900532.
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493.
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Yuliana, M.; Santoso, S.P.; Soetaredjo, F.E.; Ju, Y.H. The effect of surfactants modification on nanocrystalline cellulose for paclitaxel loading and release study. J. Mol. Liq. 2019, 282, 407–414.
- Ram, B.; Chauhan, G.S.; Mehta, A.; Gupta, R.; Chauhan, K. Spherical nanocellulose-based highly efficient and rapid multifunctional naked-eye Cr (VI) ion chemosensor and adsorbent with mild antimicrobial properties. Chem. Eng. J. 2018, 349, 146–155.
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. B Polym. Phys. 2014, 52, 791–806.
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169.
- Phanthong, P.; Reybroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43.
- Karimian, A.; Parsian, H.; Majidinia, M.; Rahimi, M.; Mir, S.M.; Kafil, H.S.; Shafiei-Irannejad, V.; Kheyrollah, M.; Ostadi, H.; Yousefi, B. Nanocrystalline cellulose: Preparation, physicochemical properties, and applications in drug delivery systems. Int. J. Biol. Macromol. 2019, 133, 850–859.
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.; Luong, J.H. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290.
- Prathapan, R.; Tabor, R.F.; Garnier, G.; Hu, J. Recent progress in cellulose nanocrystal alignment and its applications. ACS Appl. Bio Mater. 2020, 3, 1828–1844.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441.
- Kim, C.-W.; Kim, D.-S.; Kang, S.-Y.; Marquez, M.; Joo, Y.L. Structural studies of electrospun cellulose nanofibers. Polymer 2006, 47, 5097–5107.
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227.
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose–Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764.
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414.
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494.
- Huang, J.; Dufresne, A.; Lin, N. Nanocellulose: From Fundamentals to Advanced Materials; John Wiley & Sons: Hoboken, NJ, USA, 2019.
- Jozala, A.F.; De Lencastre-Novaes, L.C.; Lopes, A.M.; De Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-JR, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072.
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538.
- Wan, Y.; Wang, J.; Gama, M.; Guo, R.; Zhang, Q.; Zhang, P.; Yao, F.; Luo, H. Biofabrication of a novel bacteria/bacterial cellulose composite for improved adsorption capacity. Compos. Part A Appl. Sci. Manuf. 2019, 125, 105560.
- Gorgieva, S.; Trcek, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352.
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572.
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf. Green Technol. 2015, 2, 197–213.
- Dufresne, A. Cellulose-based composites and nanocomposites. In Monomers, Polymers, and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008.
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 40–52.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124.
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Strom, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38.
- Araki, J. Electrostatic or steric?–preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Mater. 2013, 9, 4125–4141.
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189.
- Hebeish, A. , Guthrie, T. The Chemistry and Technology of Cellulosic Copolymers; Springer Science & Business Media: Belin, Germany, 2012.
- De La Motte, H.; Hasani, M.; Brelid, H.; Westman, G. Molecular characterization of hydrolyzed cationized nanocrystalline cellulose, cotton cellulose and softwood kraft pulp using high resolution 1D and 2D NMR. Carbohydr. Polym. 2011, 85, 738–746.
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542.




