
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia Lange | + 2194 word(s) | 2194 | 2021-06-10 10:44:03 | | | |
| 2 | Dean Liu | Meta information modification | 2194 | 2021-07-09 03:35:30 | | |
Video Upload Options
Cyanine dyes are compounds that typically absorb light in the visible to near-infrared-I (NIR-I) spectrum range (750–900 nm).
1. Introduction
Cyanine dyes consist of two-terminal heterocyclic units linked by a polymethine bridge core structure (Figure 1) [1]. Over the last few years, the cyanine dyes and their derivatives were widely analyzed for the cytotoxic activity against wide spectrum of tumors. It has been reported they meet most of the requirements for being used in PDT against deep-seated cancers. Each of the main subdivisions of the dyes was described in the following paragraphs.
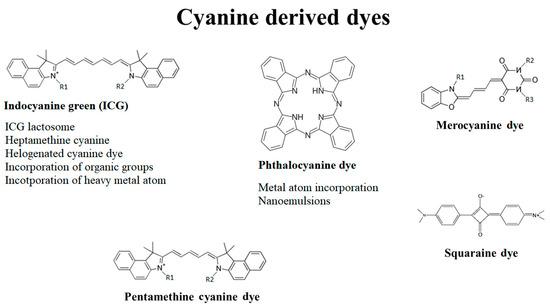
One of the most promising cyanine dye is a carbocyanine, widely known as indocyanine green (ICG), the only cyanine dye approved by the Food and Drug Administration (FDA). Due to its absorbance maximum in NIR, prominent fluorescent properties and low dark toxicity, the dye is applied to diagnose liver, cardiovascular and sentinel lymph node pathologies [2][3]. For instance, ICG-mediated PDT induced a 38% decrease in choroidal melanoma viability in six months [4]. Here, it is mostly retained in the Golgi apparatus, endoplasmic reticulum, mitochondria and lysosomes [5].
However, the dye also has several disadvantages. These include low photostability, a high level of photobleaching and no specificity towards cancer cells [6]. Of note, the energy yield from the photodynamic reaction is lower than in the PSs characterized by lower absorbance maxima [7][8].
Studies show that combining high-mass lactate-derived polymers with ICG in ICG–lactosomes (a core-shell-type polymeric micelle or “nanocarrier”) results in greater toxicity towards cancer cells in PDT than by using ICG alone. This tendency was observed in vivo on BALB/c nude mice transfected with human hepatocellular carcinoma (HCC) cell line HuH-7 [9] and gallbladder cancer NOZ cell lines [10]. In the latest case, ICG–lactosomes were effective not only as photosensitizers but also as fluorescent diagnostic agents because of their selective accumulation in cancer tissue, strengthened cytotoxicity and relatively high fluorescence.
Heptamethine cyanine dye, Cy7 is an ICG derivative that has maximum absorbance in the near-infrared range (NIR) and shows high selectivity for cancer cells. The expression of OATPs is regulated by hypoxia-inducible factor 1α (HIF1α), which may possibly explain its selectivity for tumor cells [11]. Moreover, Usama et al. proved, that Cy7 cyanine dyes form covalent albumin adducts that can generate long-lasting intratumor fluorescence due to their enhanced permeability and retention in the tumor tissue [12]. Such localization favors inducing apoptosis over necrosis, thereby minimizing the risk of uncontrolled immunological reaction provoked by necrosis of the affected tissue [13].
Cy7 is a promising photosensitizer, diagnostic agent and nanocarrier transporter. Under near-IR light this cyanine derivative might transport anti-cancer drugs exclusively to the tumor [14]. Moreover, Jiang et al. observed that Cy7 conjugated with Gemcitabine displayed relatively high residency time in tumor tissue [15]. Namely, Cy7 remains in a tumor 5–20 days in comparison to ICG, which is removed from the body within 24 h [16].
The disadvantages of heptamethine cyanine derivatives include unfavorable hydrophobicity, which leads to its aggregation in body fluids, extensive photobleaching and insufficiency in ROS production similar to ICG.
Halogenating the dye resulted in significant suppression of tumor growth. The potential of the derivative was proven in studies where PDT was more effective than a 5-FU treatment of pancreatic cancer. Namely, in a murine model transfected with a human xenograft of BxPC-3 Luc, the untreated pancreatic tumor grew to about 500% of the initial tumor size, whereas the one irradiated with iodinated cyanine IR-783 increased its volume by only about 39% [17]. The study presents PDT as a prospective neoadjuvant and palliative therapy against highly aggressive tumors.
Cao et al. modified the ICG derivative Cy7 with heavy atom iodine to form the novel NIR dye CyI [18]. They investigated the application of PDT simultaneously with photothermal therapy in the study of HepG2 cancer cells [18]. In this case, the addition of photothermal therapy (PTT) had a synergistic effect on the treatment and strengthened the suppression of tumor growth.
However, the iodinated ICG derivative CyI proved to have poor solubility and tumor-targeting abilities in clinical application [18]. To overcome the problems the dye was modified by PEGylation and the addition of hyaluronic acid, which increased solubility in water [19].
Another iodidinated cyanine dye, IR-780, accumulates preferentially in tumor tissue after an intravenous injection [20]. The derivative displays a significant in vivo ability to target tumors. The process is dependent on the cancer’s energetic metabolism, plasma membrane potential and expression of OATPs [21]. Wang et al. showed no significant difference between laser-irradiated and non-irradiated cells treated with IR-780, which is attributed to its inherent toxicity in the dark [22].
By modifying the heptamethine cyanine dye, Noh et al. developed the mitochondria-targeting photodynamic therapeutic agent MitDt-1 [23]. MitDt-1 accumulates primarily in mitochondria, thus inducing apoptosis. Also, the derivative containing triphenylphosphonium (TPP) and quaternary ammonium enhanced the dye’s solubility and selectivity for the mitochondria of the cancer cells. Moreover, the high toxicity of MitDt-1 toward cancer cells was proven in studies on MCF-7 breast cancer cells in vitro and on NCI-H460 lung cancer both in vitro and in vivo [23].
The analog of IR-780 named IR-808 (MHI-148), was designed, synthesized and screened by Tan et al. IR-808 displayed selective aggregation in tumor cells, distinct optical properties and high photostability in serum. Furthermore, IR-808 showed a significant dose-dependent phototoxic effect and distinct suppression of tumor growth after irradiation [24]. In the histopathological examination of the experimental mice, no aggregation in systemic circulation and interstitial fluids were detected.
Further modifications of IR-808 aimed to increase its water solubility, which included replacing of one of its side chains with (CH2)4SO3 in DZ-1 ICG itself has high selectivity for HCC with a high tumor to-background ratio (255:1) However, the fluorescence displayed by DZ-1 was significant and lasted longer in contrast to ICG, which allowed for the identification of smaller tumor regions. In this case, DZ-1 displayed no accumulation in liver or lung tissue, proving that DZ-1 has a higher specificity for cancer cells than ICG does.
A study by Yang et al., presented the modified heptamethine dye by the addition of 4-amino-2,2,6,6,-tetramethylpiperidine-N-oxyl [25]. Moreover, this derivative, first introduced by Jiao et al. [26], has a long triplet-state lifetime and the incorporation of sulfonic acid improves its water-solubility. Additionally, the dye prompted a significant apoptosis of the HepG2 cells after NIR irradiation and showed low dark toxicity.
Nevertheless, the introduction of the heavy atom in a cyanine dye poses a risk of enhancing dark toxicity [25] and accumulating in healthy tissues [27]. It simultaneously induced PTT and enhanced ROS production in PDT, efficiently killing A549 malignant cells [28]. Similarly, the conjugate of ICG with gold–gold sulfide, where gold also acted as an agent for PTT, presented greater stability and improved cytotoxicity towards a HeLa cell line [29]. In the study performed by Tan et al., PEGylated silver nanoparticles with a polyaniline shell acted as nanocarriers for ICG and efficiently induced hyperthermia in HeLa cancer cells.
The platinum (II) complex of heptamethine cyanine, IR797-Platin, proved to have extremely high cytotoxicity under NIR-light conditions towards C-33 A (cervical cancer) and MCF-7 breast cancer cell lines [30]. The drug’s cytotoxicity was shown by the photosensitivity of IR797 and the inhibition of DNA transcription and replication by platinum [31].
Zhao et al. synthesized the CYBF2 agent by incorporating: boron difluoride (BF2) into the core structure of cyanine [32]. BF2 reduced the dye’s electron density, resulting in the enhanced photostability. The drug accumulated in mitochondria, induced apoptosis and was efficiently absorbed by MCF-7 cells. Furthermore, the compound presented low dark toxicity and a high level of ROS burst induction.
Since extracellular cancer fluid has a high concentration of lactic acid, such a modification made it possible to visualize cancer cells and destroy them more accurately. Selectivity was proven by a higher uptake in cancer (HepG2 and HeLa) cell lines than normal cells. Also, the apoptosis following irradiation with the drug was much higher among cancer (80%) than normal (HL-7702) cells (9.4%). Interestingly, cell death was mostly the effect of hyperthermia, not photodynamic reaction.
Siriwibool et al. synthesized a pH switchable dye I2-IR783-Mpip [33] composed of IR783 and N-methylpiperazine. In acidic conditions, the color changes from blue to red, but only the red dye can absorb LED light and displays high toxicity towards HepG2 cells. Cancer cell viability in a neutral environment was about 30% and in an acidic environment decreased to 10%. In this case, death was primarily the result of a free radical production, not the photothermal effect.
who conjugated 5’-carboxyrhodamines (Rho) and heptamethine cyanine IR765 (Cy) The newly synthesized conjugate (RhoSSCy) had enhanced fluorescence in a decreased pH value and displayed high stability in pH The dye accumulated specifically in tumor cells, presenting a significant fluorescence. In the xenograft studies, the phototoxicity towards cancer was high, considerably increasing the survival rate of mice transfected with MCF-7 cells.
I range show enhanced tissue penetration. Dyes activated with NIR-II light (1000–1700 nm) were analyzed to lessen the scattering of the light by the tissue, enhance the image contrast and improve the deep-seated tumor detection. ICG and IRDye800 were shown to possess emission across NIR-I and NIR-II, marking them as promising and highly specific fluorophores for surgical procedures [7]. The photophysical mechanism of NIR-II emission relies on twisted intramolecular charge transfer (TICT).
2. Pentamethine Cyanine Dyes
Pentamethine cyanine fluorophores were designed to have tissue-specificity and localize primarily in the adrenal and pituitary glands, pancreas and lymph nodes. Their synthesis aimed to give a promising contrast agent for intraoperative imaging of glands [34]. The dyes remained active at relatively low concentrations (10 nM), and the drugs were rapidly internalized by cancer cells, which led to high ROS burst. Surprisingly, brominating the benzoindolenine did not produce any increase in free radicals.
on an ES2 ovarian carcinoma cell line [35]. The dye, which was brominated at the mesocarbon remained highly phototoxic and reduced cell line viability from 100 ± 10% to 14 ± 1% after irradiation at a 694 nm wavelength. The compound was characterized by great stability, little dark toxicity and displayed DNA-cleavage. Dye localized mainly in the cytosol and perinuclear regions, whereupon it generated hydroxyl radicals after irradiation.
3. Squaraine Dyes
Squaraine dyes possess several unique properties, such as significant fluorescence, distinct stability and absorbance wavelength maxima of 600–800 nm. The disadvantages of the dyes involve low solubility and low ROS production following PDT. ROS production was enhanced after dithiosquaraine dyes aided PDT. Despite high phytotoxicity of dithiosquaraine dyes, the compounds degraded easily and aggregated in aqueous media.
Despite low singlet oxygen production and moderate light-stability, the chemicals still remained effective. Among four synthesized dyes, a squaraine derivative with two methyl butyrate sidechains named CSBE showed an excellent phototoxic effect in vitro against seven different cancer cell lines (PC-3, MCF-7, HCT-8, A549, A549T, K562, and LoVo). CSBE effectiveness was assessed in xenograft studies, and irradiation following the injection of the dye induced tumor growth suppression. During a histological examination of the liver and kidney, no harm to healthy cells was detected.
evaluated the potency of symmetrical diiodinated benzothiazolium squaraine (SQDI) dyes in vitro on Ehrlich’s Ascites Carcinoma (EAC) cells [36]. A low concentration of the dye (0.2 mg/mL) induced 100% cytotoxicity after irradiation. An in vivo study on Swiss albino mice included the measurement of serum biochemical parameters such as SGPT, SGOT, LDH, CK and ALP after the administration of the dye through the intraperitoneal cavity. None of the parameters increased, meaning that the dye did not extend any toxicity to healthy organs.
Halogenated squaraine dyes were analyzed by Serpe et al. Their efficacy in PDT was tested in vitro on a human fibrosarcoma (HT-1080) tumor cell line [37]. Both brominated and iodinated squaraine dyes proved to induce a significant ROS generation in the first few minutes after irradiation. Despite high initial release of cytochrome c, a drastic reduction was observed 3 h after irradiation.
evaluated the potency of indolenine-based aminosquaraine cyanine dyes as photosensitizers on several cell lines: Caco-2, MCF-7, PC-3. Study revealed that, the zwitterionic dye showed high selectivity for the PC-3 cell line in comparison to the normal human cell line. Almost all aminosquaraine dyes, were specifically cytotoxic towards cancer cells and aggregated in mitochondria.
Magalhães et al. have been evaluated the efficacy of several modified zwitterionic dyes on different cancer cell lines (MCF-7, NCI-H460, HeLa, HepG2) and non-tumor porcine liver primary cell culture (PLP2) Modifications to the zwitterionic dyes were designed to increase cellular uptake by enhancing their cationic character, increase the red-shift of the dye’s absorption maximum and boost hydrophilicity. All the dyes displayed high phototoxicity towards cancer cell lines, particularly HeLa and MCF-7 cell lines, which showed the highest susceptibility to aminosquaraines. The use of the aminosquaraine analogues of benzoselenazole was also effective in cancer therapy [38], namely, the inclusion of a heavy metal, selenium, enhanced free radical production while decreasing the dye’s fluorescence emission [39].
To increase the redshift of unsymmetrical squaraine dyes, Lima et al. incorporated quinoline units into the core structure. Despite their limitations (i.e., aggregation in aqueous solution and low ROS synthesis) the new derivatives decreased their dark toxicity and presented a higher cellular uptake because of their cationic character. Although production of singlet oxygen was relatively weak, the dyes showed substantial phototherapeutic activity against breast cancer cell lines (MCF-7 and BT-474). These results were comparable to previously studied indolenine-based aminosquaraine dyes.
References
- Zhang, J.; Liu, Z.; Lian, P.; Qian, J.; Li, X.; Wang, L.; Fu, W.; Chen, L.; Wei, X.; Li, C. Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects. Chem. Sci. 2016, 7, 5995–6005.
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005, 12, 211–215.
- Bäumler, W.; Abels, C.; Karrer, S.; Weiß, T.; Messmann, H.; Landthaler, M.; Szeimies, R.M. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br. J. Cancer 1999, 80, 360–363.
- Kubicka-Trza̧ska, A.; Starzycka, M.; Romanowska-Dixon, B.; Morawski, K. Photodynamic therapy with indocyanine green for choroidal melanoma—A preliminary report. Klin. Oczna. 2003, 105, 132–135. Available online: (accessed on 1 March 2021).
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green forin vivotumor imaging. Int. J. Cancer 2016, 139, 673–682.
- Thavornpradit, S.; Usama, S.M.; Park, G.K.; Shrestha, J.P.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. QuatCy: A Heptamethine Cyanine Modification with Improved Characteristics. Theranostics 2019, 9, 2856–2867.
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 1–22.
- Englman, R.; Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970, 18, 145–164.
- Tsuda, T.; Kaibori, M.; Hishikawa, H.; Nakatake, R.; Okumura, T.; Ozeki, E.; Hara, I.; Morimoto, Y.; Yoshii, K.; Kon, M. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS ONE 2017, 12, e0183527.
- Hishikawa, H.; Kaibori, M.; Tsuda, T.; Matsui, K.; Okumura, T.; Ozeki, E.; Yoshii, K.; Liljedahl, E.; Salford, L.G.; Redebrandt, H.N. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosomes has antineoplastic effects for gallbladder cancer. Oncotarget 2019, 10, 5622–5631.
- Shi, C.; Wu, J.B.; Chu, G.C.-Y.; Li, Q.; Wang, R.; Zhang, C.; Zhang, Y.; Kim, H.L.; Wang, J.; Zhau, H.E.; et al. Heptamethine carbocyanine dye-mediated near-infrared imaging of canine and human cancers through the HIF-1α/OATPs signaling axis. Oncotarget 2014, 5, 10114–10126.
- Usama, S.M.; Park, G.K.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. Role of Albumin in Accumulation and Persistence of Tumor-Seeking Cyanine Dyes. Bioconjugate Chem. 2020, 31, 248–259.
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592.
- Gorka, A.P.; Nani, R.R.; Zhu, J.; Mackem, S.; Schnermann, M.J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159.
- Jiang, Z.; Pflug, K.; Usama, S.M.; Kuai, D.; Yan, X.; Sitcheran, R.; Burgess, K. Cyanine–Gemcitabine Conjugates as Targeted Theranostic Agents for Glioblastoma Tumor Cells. J. Med. Chem. 2019, 62, 9236–9245.
- Usama, S.M.; Lin, C.-M.; Burgess, K. On the Mechanisms of Uptake of Tumor-Seeking Cyanine Dyes. Bioconjugate Chem. 2018, 29, 3886–3895.
- Atchison, J.; Kamila, S.; Nesbitt, H.; Logan, K.A.; Nicholas, D.M.; Fowley, C.; Davis, J.; Callan, B.; McHale, A.P.; Callan, J.F. Iodinated cyanine dyes: A new class of sensitisers for use in NIR activated photodynamic therapy (PDT). Chem. Commun. 2017, 53, 2009–2012.
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated Cyanine Dyes for Fast Near-Infrared-Guided Deep Tissue Synergistic Phototherapy. ACS Appl. Mater. Interf. 2019, 11, 25720–25729.
- Sancho, D.; Joffre, O.; Keller, A.M.; Rogers, N.C.; Martínez, D.; Hernanz-Falcón, P.; Rosewell, I.; Sousa, C.R.E. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009, 458, 899–903.
- Zhang, C.; Liu, T.; Su, Y.; Luo, S.; Zhu, Y.; Tan, X.; Fan, S.; Zhang, L.; Zhou, Y.; Cheng, T.; et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials 2010, 31, 6612–6617.
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778.
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6, 27421.
- Noh, I.; Lee, D.; Kim, H.; Jeong, C.; Lee, Y.; Ahn, J.; Hyun, H.; Park, J.; Kim, Y. Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores. Adv. Sci. 2018, 5, 1700481.
- Yuan, J.; Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W. Near-infrared fluorescence imaging of prostate cancer using heptamethine carbocyanine dyes. Mol. Med. Rep. 2014, 11, 821–828.
- Yang, X.; Bai, J.; Qian, Y. The investigation of unique water-soluble heptamethine cyanine dye for use as NIR photosensitizer in photodynamic therapy of cancer cells. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2020, 228, 117702.
- Jiao, L.; Song, F.; Cui, J.; Peng, X. A near-infrared heptamethine aminocyanine dye with a long-lived excited triplet state for photodynamic therapy. Chem. Commun. 2018, 54, 9198–9201.
- Mangeolle, T.; Yakavets, I.; Marchal, S.; Debayle, M.; Pons, T.; Bezdetnaya, L.; Marchal, F. Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials 2018, 8, 572.
- Kuoabc, W.-S.; Changc, Y.-T.; Chob, K.-C.; Chiub, K.-C.; Lienb, C.-H.; Yehc, C.-S.; Chenab, S.-J. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials 2012, 33, 3270–3278.
- Ghorbani, F.; Attaran-Kakhki, N.; Sazgarnia, A. The synergistic effect of photodynamic therapy and photothermal therapy in the presence of gold-gold sulfide nanoshells conjugated Indocyanine green on HeLa cells. Photodiagnosis Photodyn. Ther. 2017, 17, 48–55.
- Mitra, K.; Lyons, C.E.; Hartman, M.C.T. A Platinum(II) Complex of Heptamethine Cyanine for Photoenhanced Cytotoxicity and Cellular Imaging in Near-IR Light. Angew. Chem. Int. Ed. 2018, 57, 10263–10267.
- Bruhn, S.L.; Toney, J.H.; Lippard, S.J. Biological Processing of DNA Modified by Platinum Compounds. Prog. Inorg. Chem. 2007, 477–516.
- Zhao, X.; Yang, Y.; Yu, Y.; Guo, S.; Wang, W.; Zhu, S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. Chem. Commun. 2019, 55, 13542–13545.
- Siriwibool, S.; Kaekratoke, N.; Chansaenpak, K.; Siwawannapong, K.; Panajapo, P.; Sagarik, K.; Noisa, P.; Lai, R.-Y.; Kamkaew, A. Near-Infrared Fluorescent pH Responsive Probe for Targeted Photodynamic Cancer Therapy. Sci. Rep. 2020, 10, 1–10.
- Owens, E.A.; Hyun, H.; Tawney, J.G.; Choi, H.S.; Henary, M. Correlating Molecular Character of NIR Imaging Agents with Tissue-Specific Uptake. J. Med. Chem. 2015, 58, 4348–4356.
- Ahoulou, E.O.; Drinkard, K.K.; Basnet, K.; Lorenz, A.S.; Taratula, O.; Henary, M.; Grant, K.B. DNA Photocleavage in the Near-Infrared Wavelength Range by 2-Quinolinium Dicarbocyanine Dyes. Molecules 2020, 25, 2926.
- Soumya, M.; Shafeekh, K.; Das, S.; Abraham, A. Symmetrical diiodinated squaraine as an efficient photosensitizer for PDT applications: Evidence from photodynamic and toxicological aspects. Chem. Interactions 2014, 222, 44–49.
- Serpe, L.; Ellena, S.; Barbero, N.; Foglietta, F.; Prandini, F.; Gallo, M.P.; Levi, R.; Barolo, C.; Canaparo, R.; Visentin, S. Squaraines bearing halogenated moieties as anticancer photosensitizers: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2016, 113, 187–197.
- Magalhães, Á.F.; Graça, V.C.; Calhelha, R.C.; Machado, I.F.; Ferreira, L.F.V.; Ferreira, I.C.F.R.; Santos, P.F. Synthesis, photochemical and in vitro cytotoxic evaluation of benzoselenazole-based aminosquaraines. Photochem. Photobiol. Sci. 2019, 18, 336–342.
- Ferreira, D.P.; Conceição, D.S.; Ferreira, V.R.A.; Graça, V.C.; Santos, P.F.; Ferreira, L.F.V. Photochemical properties of squarylium cyanine dyes. Photochem. Photobiol. Sci. 2013, 12, 1948.




