
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Foti | + 2558 word(s) | 2558 | 2021-07-02 08:20:35 | | | |
| 2 | Amina Yu | Meta information modification | 2558 | 2021-07-08 10:34:09 | | |
Video Upload Options
Monocytes are characterized by a remarkable degree of plasticity and ability to rapidly adapt to a wide range of microenvironments. A number of studies have demonstrated the importance of epigenetics in the regulation of monocyte phenotypes. Epigenetic modifications are influenced by diverse factors able to induce cell-specific changes to the environmental exposure.
1. Introduction
Neurodegeneration is an age- and disease-related process, characterized by the progressive loss and dysfunction of CNS neurons and structures.
Aging is a physiological condition of neuronal damage over time, and distinguishing neurodegeneration patterns from normal aging or related diseases poses a clear challenge [1][2]. Indeed, neurodegeneration is known to be directly mediated by cellular aging [3].
Different conditions such as oxidative stress (OS), calcium deregulation, neuroinflammation, and mitochondrial dysfunction and aggregation are all well-known drivers of neurodegeneration (Figure 1). All these processes are linked together in a long cascade of intracellular events. The oxidative stress determines mitochondrial dysfunctions at respiratory chain levels, increases cytosolic calcium, and plays a role in protein aggregation. The initial aggregation observed in Alzheimer’s disease (AD) could be a way to protect the microenvironment from oxidative damage.
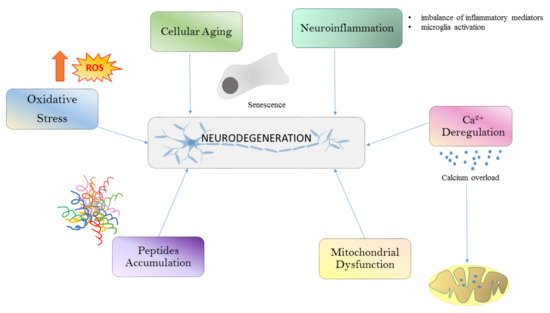
In recent years, the dynamic role of the blood–brain barrier (BBB) mediating peripheral cell migration into the brain has emerged, reflecting the contribution of peripheral systemic factors to different neurodegenerative aspects. For example, BBB damage has been observed during normal aging and becomes exaggerated in cases of cognitive impairment, regardless of the Aβ or Tau pathology [4].
Nevertheless, much remains to be clarified, and a lot of questions still remain unanswered: (i) Is neurodegeneration the consequence of neurological diseases, or are neurological diseases the consequence of neurodegeneration? (ii) To what extent does aging or specific disease impact the neurodegenerative process?
2. Epigenome Regulation of Monocytes Plasticity in Neurodegeneration
A number of studies have demonstrated the importance of epigenetics in the regulation of monocyte phenotypes [5]. Epigenetic modifications are influenced by diverse factors able to induce cell-specific changes to the environmental exposure. Since monocytes circulate in the blood, and their epigenome maybe influenced by the presence of diverse molecules such as food-derived metabolites, and in case of pathological conditions also by different inflammatory mediators. To date epigenetic changes, include the following categories: (i) DNA methylation, (ii) histone modifications and (iii) non-coding RNA.
In general, DNA methylation is associated with transcriptional repression and is related to the transfer of a methyl group to the cytosine base of the DNA by DNA methyltransferases (DNMTs) to form 5-methyl-citosine (5 mC). Histone modifications regulate cellular phenotypes by adding or removing the acetyl or methyl group in histone proteins; these activities are regulated by acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. Histone acetylation is linked to transcriptional activity whereas histone deacetylation is associated with transcriptional repression [6]. Similarly, methylation and demethylation of histones is achieved by histone methyltransferases (HMTs) and histone demethylases (HDMs), respectively.
Epigenetic changes have also been implicated in the pathogenesis of a number of neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, or amyotrophic lateral sclerosis (ALS) [7][8][9]; nevertheless, a detailed role of monocyte epigenetics in these diseases is still missing. Regarding multiple sclerosis (MS), to date, small number of studies have addressed the role of epigenetically mediated changes in blood of MS patients. Methylation profiles of mainly CD4+, CD8+T cells, B cells, monocytes, and cell-free plasma DNA were reported, and the most interesting findings were related to hypomethylation on the IL17A promoter region, which is known to correlate with Th17 cell lineage generation and a decrease in the methylation pattern located in the HLA-DRB1 gene suggesting that the DRB1 haplotype may influence the association observed between the methylation level at DRB1 CpGs and MS risk [10][11][12].
Monocyte epigenomics was described in one study [13]. The authors found that B cells and monocyte methylation profiles were the most different between relapsing remitting multiple sclerosis (RRMS) and healthy controls. No significant differences were described for CD4 and CD8 T cells.
Usually, non-coding RNA (ncRNA) are grouped under the epigenetic mechanism as they have an important role in regulating coding and non-coding regions of the genome beside a direct regulation of the gene expression. There are several subtypes of long and short ncRNA species, many of which are involved in regulation of gene expression, and can be further grouped according to their genomic origins and biogenic processes. MicroRNAs profiling in MS has also been extensively studied in peripheral blood mononuclear cells, whole blood, lymphocytes, and cell-free plasma to elucidate their role in MS pathogenesis. All these data strongly suggest the need to define strategies for the development of a precision medicine approach so that genomics and/or epigenomics analysis will help to define the precise pathogenic mechanisms operating within a subgroup of well-defined MS patients’ clinical stage.
Finally, microRNA can also be released into membrane-bound vesicles (also referred to as extracellular vesicles, or EVs) and several studies have reported a role of EVs in neurodegeneration. It was suggested that monocytes plasticity can also be modulated by microRNA molecules that are present within EVs. Indeed, in vitro experiments showed that endothelial-derived EVs promoted monocytes activation by enhancing monocytes migration through an endothelial monolayer [14]. In addition, a recent study also showed a reduction of monocyte-derived EVs in samples obtained from patients after one year of fingolimod treatment suggesting that EVs were indeed implicated through the modulation of monocyte activity with the mechanisms of action of immunomodulatory treatments [15][16].
In summary, evidence suggests that epigenetics play a role in monocyte phenotypes. Thus, it will be important to understand the type of mechanisms that drive monocyte diversity and plasticity in the context of neurodegeneration. Dysregulated epigenetic changes may contribute to the persistence of the disease, and therefore, a future challenge will be to understand how to modulate these modifications to develop novel treatments for neurodegenerative diseases.
3. Monocytes Migration into the Brain during Neurodegeneration
The mechanisms by which leukocytes pass through the barriers of the brain and their role in progression of neurological diseases remain yet to be fully elucidated. Although it is now accepted that the CNS undergoes immune surveillance at meningeal level [17], the mechanisms involved in immune cell trafficking in CNS remain poorly understood. The myeloid compartment in the CNS is composed of tissue-resident microglia found in the brain parenchyma and additional myeloid cells including DCs, monocytes, and granulocytes in the meningeal area.
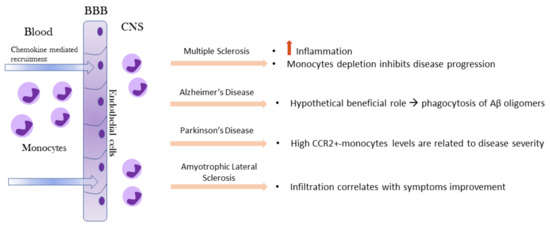
Under physiological conditions, monocytes are not detectable in brain or spinal cord parenchyma but only observed in the meninges [18]. Monocyte functions in the brain have been investigated primarily under pathological conditions. The recruitment of blood monocytes to the CNS following infection, injury, or an inflammatory response is often observed in neurologic disorders. After injury or during specific disease processes, the brain becomes highly permeable to circulating peripheral cells, including monocytes (Figure 3).
A recent study conducted on human brains suggested that granulocyte–macrophage colony-stimulating factor (GM-CSF) may play a role, especially during autoimmune diseases, such as MS [19]. Beside GM-CSF and the CCL2-CCR2 axis, the CD49e (α5 integrin) was reported to play a role in monocytes brain migration. It was shown that α5 integrin is expressed only on the peripheral monocyte populations but not on CNS-resident myeloid cell populations. Treatment with α5 integrin antibody significantly reduced the experimental autoimmune encephalomyelitis (EAE) disease severity and therefore provides a strong rationale for a novel therapeutic approach that specifically targets and inhibits monocyte trafficking into the CNS thus leading to fewer deleterious side effects observed with drugs that block T lymphocytes migration [20].
Leukocyte migration to the cerebrospinal fluid (CSF) and Peripheral administration of the TLR2 ligand, Pam3Cys, induced marked infiltration of neutrophils and monocytes to the CSF and brain of neonatal mice. These studies suggest novel mechanisms of leukocyte migration to the brain and potential therapeutic targets to ameliorate neuroinflammation induced by meningitis or other CNS pathologies [21]. Mechanistically, the inhibition targeting CD40-TRAF6 signaling is mediated by the limitation of ROS production in monocytes and consequently a reduce migration of the cells across an in vitro BBB [22].
In the EAE rodent model of MS, gene-expression profiles indicated that infiltrating monocytes are highly inflammatory compared to microglia [23]. A correlation between monocyte infiltration into the CNS and progression to the paralytic stage of the disease has been shown: depletion of monocytes was shown to significantly inhibit both disease initiation and disease progression in EAE mice [24].
In AD, monocytes are recruited at the site of Aβ deposits and in the inflammatory microenvironment around them. In fact, after migration to injured brain, monocytes can differentiate into macrophages and phagocytize protein aggregates such as Aβ [25]. MCP-1, which is produced by Aβ-induced activated microglial cells [26], triggers the mobilization of pro-inflammatory monocytes in the inflamed brain through the MCP-1 receptor CCR2 [27].
The first general evidence of immune dysregulation in PD patients was shown by measurement of elevated levels of cytokines (IL-2, IL-4, IL-6, IL-10, TNFα) in the serum and peripheral blood mononuclear cells were suspected to contribute to this peripheral cytokine elevation. For what concern monocytes brain migration, direct invasion of peripheral monocytes into the CNS has been demonstrated in an animal model for PD [28]. In humans, a strong upregulation of CCR2 on classical monocytes in Parkinson’s patients was detected whereas the percentage of these cells was specifically downregulated, suggesting that this cellular population may have migrated to the inflamed brain. Indeed, it is known that upregulation of CCR2 is essential for monocyte recruitment in inflamed tissue [29].
Similarly, in ALS, circulating human monocytes were found to be dysregulated regarding function, gene expression and subset constitution. Monocytes from ALS patients exhibited an altered adhesion capacity, which indicated a changed migratory potential. The exact role of CNS-infiltrating monocytes in ALS had remained ambiguous so far, but the mouse model of the disease (SOD1G93A tg mice) implies a role of peripheral monocytes early in the disease [30]. CNS infiltration of peripheral monocytes correlates with improved motor neuron survival in a genetic ALS mouse model [31].
Therefore, we can conclude that monocyte infiltration in brain may be both beneficial or harmful and that the exact role of these cells in different disease contexts needs further investigation. Unfortunately, we are not yet able to distinguish resident microglia from infiltrated monocytes with absolute certainty, especially in humans, and therefore we cannot rule out at the moment, the exact role of monocytes in the brain inflammatory response.
4. Monocytes Plasticity in Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a debilitating neurodegenerative disease with reported immune dysregulations [31][32][33][34]. Different studies have reported that peripheral immune system cells are functionally altered, especially those with myeloid lineage [35][36][37][38][39]. One of the most challenging factors in most neurodegenerative disease, including ALS, is their heterogeneity of clinical features which render it challenging to identify factors that alone may explain all the pathological mechanisms that eventually are operating in the disease. Any given cohort of patients varies in terms of severity, progression, site of onset, degree of respiratory involvement, and degree of upper or lower motor neuron involvement [40].
Within the myeloid population so far studied, monocytes have been reported to play a role [37]. ALS patients with distinct clinical features have differential monocyte cell subset distribution, for example patients with greater disease severity, as determined by a lower revised amyotrophic lateral sclerosis functional rating scale score, showed a reduced non-classical monocyte subset whereas patients with greater bulbar involvement had a reduction in the proportion of classical, intermediate, and non-classical monocyte populations. On the same line, CD16 expression in neutrophils increased in patients with greater disease severity and a faster rate of disease progression, whereas HLA-DR expression in all monocyte populations was elevated in patients with greater respiratory impairment [41].
Previous literature reporting on immune cell frequencies and marker expression has not revealed consistent findings again probably because of the heterogeneity of patients and methodological variations between studies. We should always keep in mind that each cohort population under study is unique and therefore what we observe in one cohort may not be valid for others. To avoid confusing factors, guidelines on immunophenotyping in whole blood should be adopted to be able to compare results from different studies [42][43].
ALS monocytes skewing toward a proinflammatory state have been investigated by RNAseq analysis. Gene expression profiles were studied in 23 ALS monocytes compared to 10 healthy control individuals, and demonstrated that monocytes isolated from patients with ALS expressed a unique gene profile associated with proinflammatory immune responses. These findings were validated through qRT-PCR in an additional cohort confirming the higher mRNA expression values in monocytes of ALS patients. These results were obtained from monocytes isolated by negative selection, which could have a lower impact on cellular activation compared to positive selection.
ALS peripheral monocytes produce more pro-inflammatory cytokines when stimulated with LPS and IFNγ to differentiate into M1 phenotype suggesting that ALS monocytes are functionally altered, which could explain an increased cytotoxicity once they arrive into the CNS [44]. The functional alteration in ALS monocytes was also demonstrated by examining the adhesion capacity of ALS monocytes, which suggested a change in their migratory capacity. Indeed, the number of adhering monocytes is higher in ALS than HCs after LPS stimulation, and monocyte transmigration is known to be preceded by extravasation and adherence to the vessel walls [38].
Further supporting the role of monocytes in ALS pathogenesis is the finding that the transactive response DNA-binding protein 43 (TDP-43) is accumulated in a subgroup of ALS cases again underlying the possibility that different mechanisms of disease are operating in different cohorts of patients [45]. These issues should be further deeply investigated as we hypothesize that they will explain the great clinical heterogeneity we observe in ALS and in general in neurodegenerative diseases.
Finally, it will be important to clearly distinguish microglia from peripheral blood-derived monocytes infiltrating the brain. Recently, the CD169/Siglec-1 molecule was suggested as a marker for monocytes in the CNS, because it is not expressed in resident microglia [37][46]. By using this molecule, it was possible to show that CD169+cells were significantly higher in lumbar spinal cords of 10 ALS patients. The ALS CD169+monocytes were shown to have a decreased diameter, and to be located within the tissue (80.2%) with only a small percentage within the perivascular space (19.8%)
Interestingly, in SODG93AALS mouse model, immunomodulatory treatment increased the CD169+cells that correlated with the enhancement of motor neuron survival, suggesting that monocyte invasion at least in this experimental model, acted as a neuroprotective in the early stage studied.
In conclusion, monocytes may have a role in ALS pathogenesis therefore, it can be hypothesized that suppression of their pro-inflammatory phenotype may provide a new therapeutic option for ALS. Nevertheless, to reach this end point, there is a need to further expand our knowledge at the monocyte single cell level to precisely identify the specific monocyte subset infiltrating the brain that may exert a pathogenic as well as protective effect in this disease.
References
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216.
- Guerra-Araiza, C.; Alvarez-Mejía, A.L.; Sanchez-Torres, S.; Farfan-García, E.; Mondragon-Lozano, R.; Pinto-Almazán, R.; Salgado-Ceballos, H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free. Radic. Res. 2013, 47, 451–462.
- Thal, D.R.; Del Tredici, K.; Braak, H. Neurodegeneration in Normal Brain Aging and Disease. Sci. Aging Knowl. Environ. 2004, 2004, pe26.
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276.
- Liu, Y.; Reynolds, L.M.; Ding, J.; Hou, L.; Lohman, K.; Young, T.; Cui, W.; Huang, Z.; Grenier, C.; Wan, M.; et al. Blood monocyte transcriptome and epigenome analyses reveal loci associated with human atherosclerosis. Nat. Commun. 2017, 8, 393.
- Sterner, D.E.; Berger, S.L. Acetylation of Histones and Transcription-Related Factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459.
- Marques, S.C.; Oliveira, C.R.; Pereira, C.M.; Outeiro, T.F. Epigenetics in neurodegeneration: A new layer of complexity. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2011, 35, 348–355.
- Marques, S.; Outeiro, T.F. Epigenetics in Parkinson’s and Alzheimer’s diseases. Subcell. Biochem. 2013, 61, 507–525.
- Calió, M.L.; Henriques, E.; Siena, A.; Bertoncini, C.R.A.; Gil-Mohapel, J.; Rosenstock, T.R. Mitochondrial Dysfunction, Neurogenesis, and Epigenetics: Putative Implications for Amyotrophic Lateral Sclerosis Neurodegeneration and Treatment. Front. Neurosci. 2020, 14, 679.
- Graves, M.C.; Benton, M.; Lea, R.A.; Boyle, M.; Tajouri, L.; Macartney-Coxson, D.; Scott, R.J.; Lechner-Scott, J. Methylation differences at the HLA-DRB1 locus in CD4+ T-Cells are associated with multiple sclerosis. Mult. Scler. J. 2014, 20, 1033–1041.
- Rhead, B.; Brorson, I.S.; Berge, T.; Adams, C.; Quach, H.; Moen, S.M.; Berg-Hansen, P.; Celius, E.G.; Sangurdekar, D.P.; Bronson, P.G.; et al. Increased DNA methylation of SLFN12 in CD4+ and CD8+ T cells from multiple sclerosis patients. PLoS ONE 2018, 13, e0206511.
- Maltby, V.E.; Graves, M.C.; Lea, R.A.; Benton, M.C.; Sanders, K.A.; Tajouri, L.; Scott, R.J.; Lechner-Scott, J. Genome-wide DNA methylation profiling of CD8+ T cells shows a distinct epigenetic signature to CD4+ T cells in multiple sclerosis patients. Clin. Epigenet. 2015, 7, 118.
- Ewing, E.; Kular, L.; Fernandes, S.J.; Karathanasis, N.; Lagani, V.; Ruhrmann, S.; Tsamardinos, I.; Tegner, J.; Piehl, F.; Gomez-Cabrero, D.; et al. Combining evidence from four immune cell types identifies DNA methylation patterns that implicate functionally distinct pathways during Multiple Sclerosis progression. EBioMedicine 2019, 43, 411–423.
- Jy, W.; Minagar, A.; Jimenez, J.J.; Sheremata, W.A.; Mauro, L.M.; Horstman, L.L.; Bidot, C.; Ahn, Y.S. Endothelial microparticles (EMP) bind and activate monocytes: Elevated EMP-monocyte conjugates in multiple sclerosis. Front. Biosci. 2004, 9, 3137–3144.
- Sáenz-Cuesta, M.; Alberro, A.; Muñoz-Culla, M.; Osorio-Querejeta, I.; Fernandez-Mercado, M.; Lopetegui, I.; Tainta, M.; Prada, Á.; Castillo-Triviño, T.; Falcón-Pérez, J.M.; et al. The First Dose of Fingolimod Affects Circulating Extracellular Vesicles in Multiple Sclerosis Patients. Int. J. Mol. Sci. 2018, 19, 2448.
- Dalla Costa, G.; Finardi, A.; Garzetti, L.; Carandini, T.; Comi, G.; Martinelli, V.; Furlan, R. Disease-modifying treatments modulate myeloid cells in multiple sclerosis patients. Neurol. Sci. 2018, 39, 373–376.
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635.
- Giladi, A.; Wagner, L.K.; Li, H.; Dörr, D.; Medaglia, C.; Paul, F.; Shemer, A.; Jung, S.; Yona, S.; Mack, M.; et al. Cxcl10(+) monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 2020, 21, 525–534.
- Vogel, D.Y.S.; Kooij, G.; Heijnen, P.D.A.M.; Breur, M.; Peferoen, L.A.N.; Van Der Valk, P.; De Vries, H.E.; Amor, S.; Dijkstra, C.D. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur. J. Immunol. 2015, 45, 1808–1819.
- Ajami, B.; Samusik, N.; Wieghofer, P.; Ho, P.P.; Crotti, A.; Bjornson, Z.; Prinz, M.; Fantl, W.J.; Nolan, G.P.; Steinman, L. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 2018, 21, 541–551.
- Mottahedin, A.; Svedin, P.; Nair, S.; Mohn, C.-J.; Wang, X.; Hagberg, H.; Ek, J.; Mallard, C. Systemic activation of Toll-like receptor 2 suppresses mitochondrial respiration and exacerbates hypoxic–ischemic injury in the developing brain. J. Cereb. Blood Flow Metab. 2017, 37, 1192–1198.
- Aarts, S.A.B.M.; Seijkens, T.T.P.; Kusters, P.J.H.; Van Der Pol, S.M.A.; Zarzycka, B.; Heijnen, P.D.A.M.; Beckers, L.; den Toom, M.; Gijbels, M.J.J.; Boon, L.; et al. Inhibition of CD40-TRAF6 interactions by the small molecule inhibitor 6877002 reduces neuroinflammation. J. Neuroinflamm. 2017, 14, 105.
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014, 211, 1533–1549.
- Ajami, B.; Bennett, J.L.; Krieger, C.; McNagny, K.M.; Rossi, F.M.V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011, 14, 1142–1149.
- Malm, T.; Koistinaho, M.; Muona, A.; Magga, J.; Koistinaho, J. The role and therapeutic potential of monocytic cells in Alzheimer’s disease. Glia 2010, 58, 889–900.
- Thériault, P.; ElAli, A.; Rivest, S. The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 41.
- Naert, G.; Rivest, S. A deficiency in CCR2+ monocytes: The hidden side of Alzheimer’s disease. J. Mol. Cell Biol. 2013, 5, 284–293.
- Depboylu, C.; Stricker, S.; Ghobril, J.-P.; Oertel, W.H.; Priller, J.; Höglinger, G.U. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp. Neurol. 2012, 238, 183–191.
- Funk, N.; Wieghofer, P.; Grimm, S.; Schaefer, R.; Bühring, H.-J.; Gasser, T.; Biskup, S. Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov. Disord. 2013, 28, 392–395.
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 2015, 77, 75–99.
- Zondler, L.; Feiler, M.S.; Freischmidt, A.; Ruf, W.P.; Ludolph, A.C.; Danzer, K.M.; Weishaupt, J.H. Impaired activation of ALS monocytes by exosomes. Immunol. Cell Biol. 2016, 95, 207–214.
- Zhang, R.; Gascon, R.; Miller, R.G.; Gelinas, D.F.; Mass, J.; Hadlock, K.; Jin, X.; Reis, J.; Narvaez, A.; McGrath, M.S. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2005, 159, 215–224.
- Babu, G.N.; Kumar, A.; Chandra, R.; Puri, S.K.; Kalita, J.; Misra, U.K. Elevated Inflammatory Markers in a Group of Amyotrophic Lateral Sclerosis Patients from Northern India. Neurochem. Res. 2008, 33, 1145–1149.
- Zhao, W.; Beers, D.R.; Appel, S.H. Immune-mediated Mechanisms in the Pathoprogression of Amyotrophic Lateral Sclerosis. J. Neuroimmune Pharmacol. 2013, 8, 888–899.
- Graves, M.C.; Fiala, M.; Dinglasan, L.A.V.; Liu, N.Q.; Sayre, J.; Chiappelli, F.; Van Kooten, C.; Vinters, H.V. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Mot. Neuron Disord. 2004, 5, 213–219.
- Henkel, J.S.; Engelhardt, J.I.; Siklós, L.; Simpson, E.P.; Kim, S.H.; Pan, T.; Goodman, J.C.; Siddique, T.; Beers, D.R.; Appel, S.H. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2004, 55, 221–235.
- Butovsky, O.; Siddiqui, S.; Gabriely, G.; Lanser, A.J.; Dake, B.; Murugaiyan, G.; Doykan, C.E.; Wu, P.M.; Gali, R.R.; Iyer, L.; et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Investig. 2012, 122, 3063–3087.
- Zondler, L.; Müller, K.; Khalaji, S.; Bliederhäuser, C.; Ruf, W.P.; Grozdanov, V.; Thiemann, M.; Fundel-Clemes, K.; Freischmidt, A.; Holzmann, K.; et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016, 132, 391–411.
- Zhao, W.; Beers, D.R.; Hooten, K.G.; Sieglaff, D.H.; Zhang, A.; Kalyana-Sundaram, S.; Traini, C.M.; Halsey, W.S.; Hughes, A.M.; Sathe, G.M.; et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes. JAMA Neurol. 2017, 74, 677–685.
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172.
- McGill, R.B.; Steyn, F.J.; Ngo, S.T.; Thorpe, K.A.; Heggie, S.; Ruitenberg, M.J.; Henderson, R.D.; McCombe, P.A.; Woodruff, T.M. Monocytes and neutrophils are associated with clinical features in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, fcaa013.
- Lundahl, J.; Halldén, G.; Hallgren, M.; Sköld, C.M.; Hed, J. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J. Immunol. Methods 1995, 180, 93–100.
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80.
- Du, Y.; Zhao, W.; Thonhoff, J.R.; Wang, J.; Wen, S.; Appel, S.H. Increased activation ability of monocytes from ALS patients. Exp. Neurol. 2020, 328, 113259.
- De Marco, G.; Lomartire, A.; Calvo, A.; Risso, A.; De Luca, E.; Mostert, M.; Mandrioli, J.; Caponnetto, C.; Borghero, G.; Manera, U.; et al. Monocytes of patients with amyotrophic lateral sclerosis linked to gene mutations display altered TDP-43 subcellular distribution. Neuropathol. Appl. Neurobiol. 2017, 43, 133–153.
- Gao, L.; Brenner, D.; Llorens-Bobadilla, E.; Saiz-Castro, G.; Frank, T.; Wieghofer, P.; Hill, O.; Thiemann, M.; Karray, S.; Prinz, M.; et al. Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice. J. Exp. Med. 2015, 212, 469–480.




