
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoshikazu Nakamura | + 947 word(s) | 947 | 2021-07-06 12:18:41 | | | |
| 2 | Vicky Zhou | Meta information modification | 947 | 2021-07-08 05:16:35 | | |
Video Upload Options
RBM-007 is an anti-FGF2 aptamer composed of 37 nucleotides, whose ribose 2′ positions are modified to resist ribonucleases, in addition to being 5′-PEGylated and 3′-conjugated with an inverted dT to confer an advantageous pharmacokinetic profile. RBM-007 binds strongly and specifically to FGF2 and does not cross-react with other FGF family proteins or heparin-binding proteins, blocking the interaction between human FGF2 and its receptors FGFR1 through FGFR4. The dissociation constant (KD) of the non-PEGylated form of RBM-007 to human FGF2 protein is 2 pM, compared to 5, 7, and 27 pM in rat, mouse, and rabbit protein, respectively, showing the high affinity of RBM-007 for different FGF2s regardless of the species difference.
1. Introduction
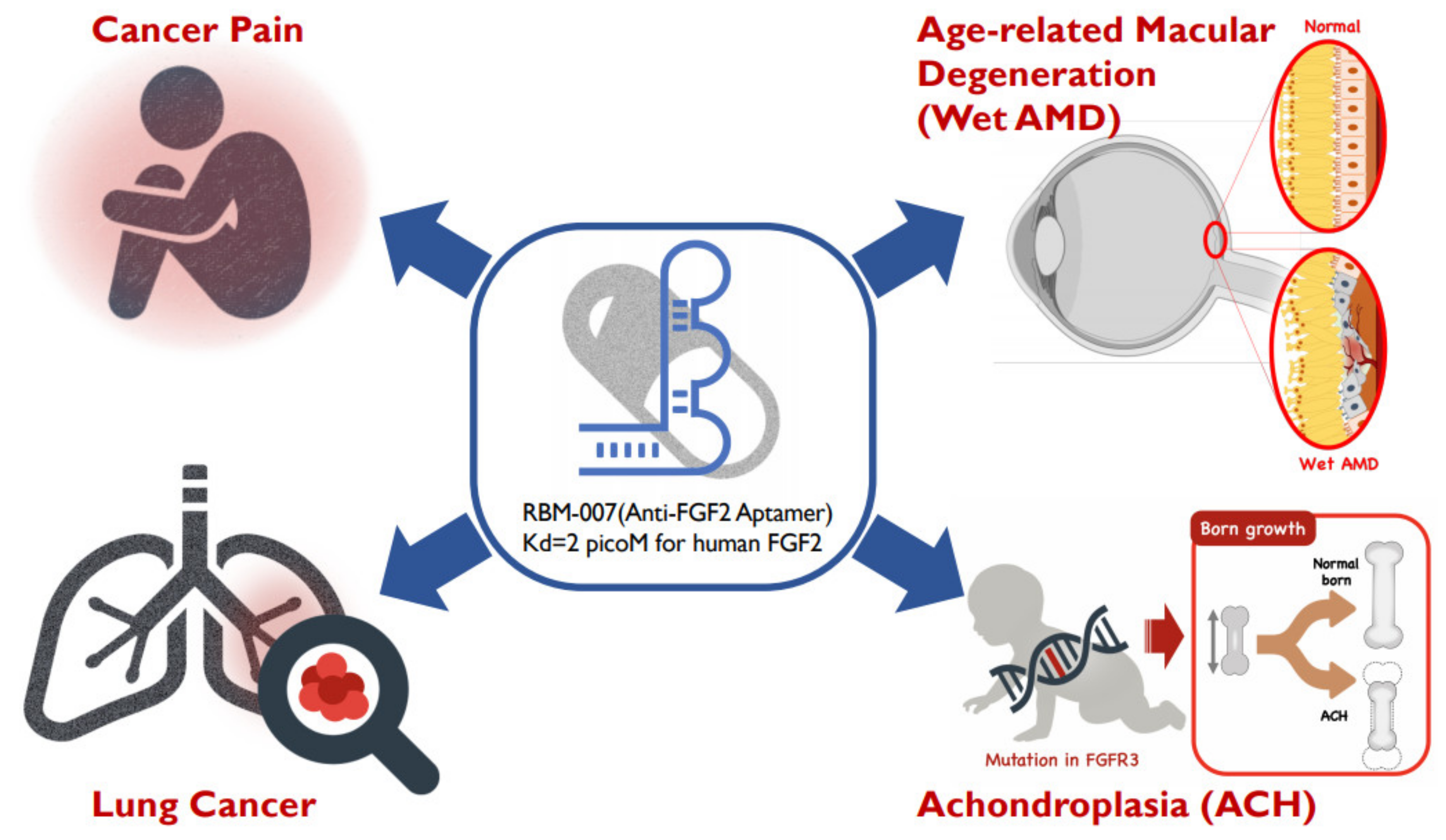 Figure 1. Diverse therapeutic applications of RBM-007 (anti-FGF2 aptamer).
Figure 1. Diverse therapeutic applications of RBM-007 (anti-FGF2 aptamer).2. Facts about RBM-007
References
- Krejci, P.; Prochazkova, J.; Bryja, V.; Kozubik, A.; Wilcox, W.R. Molecular pathology of the fibroblast growth factor family. Hum. Mutat. 2011, 30, 1245–1255.
- Marie, P.J.; Miraoui, H.; Severe, N. FGF/FGFR signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123.
- Okada-Ban, M.; Thiery, J.P.; Jouanneau, J. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 2000, 32, 263–267.
- Eda, H.; Aoki, K.; Marumo, K.; Fujii, K.; Ohkawa, K. FGF-2 signaling induces downregulation of TAZ protein in osteoblastic MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 2008, 366, 471–475.
- Kawaguchi, H.; Nakamura, K.; Tabata, Y.; Ikada, Y.; Aoyama, I.; Anzai, J.; Nakamura, T.; Hiyama, Y.; Tamura, M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. J. Clin. Endocrinol. Metab. 2001, 86, 875–880.
- Nakagawa, N.; Yasuda, H.; Yano, K.; Mochizuki, S.; Kobayashi, N.; Fujimoto, H.; Shima, N.; Morinaga, T.; Chikazu, D.; Kawaguchi, H.; et al. Basic fibroblast growth factor induces osteoclast formation by reciprocally regulating the production of osteoclast differentiation factor and osteoclastogenesis inhibitory factor in mouse osteoblastic cells. Biochem. Biophys. Res. Commun. 1999, 265, 158–163.
- Kawaguchi, H.; Chikazu, D.; Nakamura, K.; Kumegawa, M.; Hakeda, Y. Direct and indirect actions of fibroblast growth factor 2 on osteoclastic bone resorption in cultures. J. Bone Miner. Res. 2000, 15, 466–473.
- Matsuzaki, K.; Yoshitake, Y.; Matuo, Y.; Sasaki, H.; Nishikawa, K. Monoclonal antibodies against heparin-binding growth factor II/basic fibroblast growth factor that block its biological activity: Invalidity of the antibodies for tumor angiogenesis. Proc. Natl. Acad. Sci. USA 1989, 86, 9911–9915.
- Rege, A.A.; Bjercke, R.J.; Erichsen, D.; Owens, R.; Stephan, C.C.; Brock, T.A. Development of novel monoclonal antibodies for the analysis of functional sites in FGF-2. Growth Factors 1999, 16, 161–169.
- Kuhn, H.; Kopff, C.; Konrad, J.; Riedel, A.; Gessner, C.; Wirtz, H. Influence of basic fibroblast growth factor on the proliferation of non-small cell lung cancer cell lines. Lung Cancer 2004, 44, 167–174.
- Hori, A.; Sasada, R.; Matsutani, E.; Naito, K.; Sakura, Y.; Fujita, T.; Kozai, Y. Suppression of solid tumor growth by immunoneutralizing monoclonal antibody against human basic fibroblast growth factor. Cancer Res. 1991, 51, 6180–6184.
- Wang, L.; Park, H.; Chhim, S.; Ding, Y.; Jiang, W.; Queen, C.; Kim, K.J. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol. Cancer Ther. 2012, 11, 864–872.
- Jin, L.; Nonaka, Y.; Miyakawa, S.; Fujiwara, M.; Nakamura, Y. Dual therapeutic action of a neutralizing anti-FGF2 aptamer in bone diseases and bone cancer pain. Mol. Ther. 2016, 24, 1974–1986.
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550.
- Ellington, A.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822.
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510.
- Nomura, Y.; Sugiyama, S.; Sakamoto, T.; Miyakawa, S.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Nakamura, Y.; et al. Conformational plasticity of RNA for target recognition as revealed by the 2.15 Å crystal structure of a human IgG-aptamer complex. Nucl. Acids Res. 2010, 38, 7822–7829.
- Nakamura, Y. Aptamers as therapeutic middle molecules. Biochimie 2018, 145, 22–33.
- Matsuda, Y.; Nonaka, Y.; Futakawa, S.; Imai, H.; Akita, K.; Nishihata, T.; Fujiwara, M.; Ali, Y.; Bhisitkul, R.B.; Nakamura, Y. Anti-angiogenic and anti-scarring dual action of an anti-fibroblast growth factor 2 aptamer in animal models of retinal disease. Mol. Ther. Nucl. Acids 2019, 17, 819–828.
- Oladipupo, S.S.; Smith, C.; Santeford, A.; Park, C.; Sene, A.; Wiley, L.A.; Osei-Owusu, P.; Hsu, J.; Zapata, N.; Liu, F.; et al. Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc. Natl. Acad. Sci. USA 2014, 111, 13379–13384.
- Dong, Z.; Santeford, A.; Ban, N.; Lee, T.J.; Smith, C.; Smith, C.; Ornitz, D.M.; Apte, R.S. FGF2-induced STAT3 activation regulates pathologic neovascularization. Exp. Eye Res. 2019, 187, 107775.




