Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Elizabeth Cawood | + 990 word(s) | 990 | 2021-06-24 08:04:43 | | | |
| 2 | Peter Tang | Meta information modification | 990 | 2021-07-07 11:23:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cawood, M.E. Phytochemicals in Daucus carota. Encyclopedia. Available online: https://encyclopedia.pub/entry/11770 (accessed on 08 February 2026).
Cawood ME. Phytochemicals in Daucus carota. Encyclopedia. Available at: https://encyclopedia.pub/entry/11770. Accessed February 08, 2026.
Cawood, Maria Elizabeth. "Phytochemicals in Daucus carota" Encyclopedia, https://encyclopedia.pub/entry/11770 (accessed February 08, 2026).
Cawood, M.E. (2021, July 07). Phytochemicals in Daucus carota. In Encyclopedia. https://encyclopedia.pub/entry/11770
Cawood, Maria Elizabeth. "Phytochemicals in Daucus carota." Encyclopedia. Web. 07 July, 2021.
Copy Citation
Carrots are a multi-nutritional food source. They are an important root vegetable, rich in natural bioactive compounds, which are recognised for their nutraceutical effects and health benefits.
carrot
phenolic compounds
carotenoids
polyacetylenes
ascorbic acid
human health
1. Introduction
Fruits and vegetables are rich sources of nutrients that contain phytochemicals (also known as bioactive compounds), which are recognised for their nutraceutical effects and health benefits [1]. The cultivated carrot (Daucus carota L.) is one of the most important vegetable plants in the world because of its high yield potential and use as fresh or processed product. With an annual world production (carrots and turnips) of >428 million tons and a total growing area of about 11.5 million hectares [2], carrots rank among the top 10 vegetable crops in the world [3]. They play a major role in human nutrition, because of their high dietary value and good storage attributes [4][5]. Phytochemicals contribute to the dietary value of carrots and comprise mainly four types; namely, phenolic compounds, carotenoids, polyacetylenes, and ascorbic acid.
2. The Carrot Plant
The edible carrot, Daucus carota, is the most important root vegetable plant grown worldwide and it is part of the Apiaceae family [6]. The carrot consists mainly of two parts, the stem and the root, and most of the root consists of the peel (periderm), a pulpy outer cortex (phloem), and an inner core (xylem). Figure 1 shows the carrot root’s anatomy. Cultivated carrots have orange, reddish, purple, black, or yellow roots. The most commonly eaten part of the carrot plant is the root, though the stems and leaves are eaten as well, so the present review is in regard to the root, unless otherwise indicated.
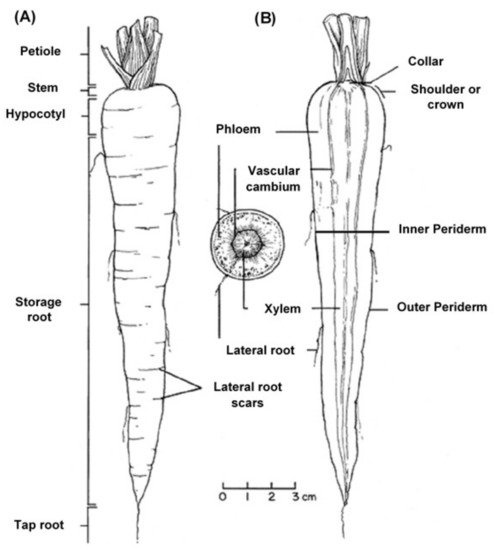
Figure 1. Carrot root anatomy: (A) longitudinal; (B) cross-section, showing the periderm, phloem, and xylem (www.carrotmuseum.co.uk).
3. Phenolic Compounds
Phenolic compounds constitute one of the most ubiquitous groups of plant metabolites and are an integral part of both human and animal diets. The role of polyphenols in the prevention of degenerative diseases, like cancer, cardiovascular diseases, and neurodegenerative diseases has been reported. Interest in food phenolics has increased greatly over the past two decades, owing to their antioxidant capacity and their function as a defence against oxidative stress caused by excess reactive oxygen species [7].
Phenolic compounds are secondary plant metabolites, mainly composed of an aromatic ring bearing one or more hydroxyl groups, playing a crucial role in counteracting various type of stress (ultraviolet irradiation, aggression by pathogens, parasites, and plant predators), and contributing to the organoleptic properties of plants and plant-derived food [8][9][10][11]. Phenolic compounds can be divided into different subgroups, such as phenolic acids, flavonoids, tannins, lignans, stilbenoids, and curcuminoids. It has been reported that carrots are rich in phenolic acids, such as p-hydroxybenzoic, caffeic, and chlorogenic, as well as in anthocyanins, a class of flavonoids (Figure 2) [12].
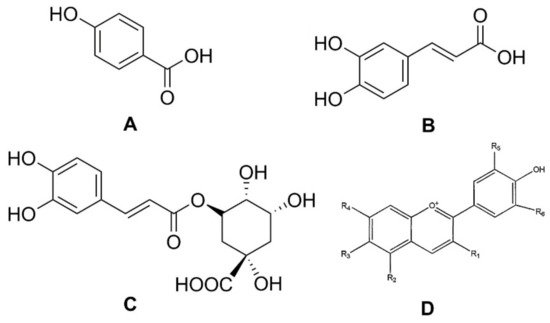
Figure 2. Structures of phenolic acids: (A) p-hydroxybenzoic acid; (B) caffeic acid; (C) chlorogenic acid; and (D) the basic chemical structure of anthocyanins.
Isocoumarins and phenolic acids are the potentially bitter compounds found in carrot peels. Czepa and Hofmann [13] reported that the bitter taste in carrots is caused by terpenoids and water-soluble phenolics. Therefore, their presences can be used as biological markers to assess the quality of fruits and vegetables during postharvest operations [14].
4. Carotenoids
Carotenoids are a group of isoprenoid molecules present in all photosynthetic plants, including carrots. Some non-photosynthetic fungi and bacteria also possess carotenoids. Carotenoids are acyclic or have five or six C rings on one or both ends of the molecule [14]. Several conjugated double bonds of a polyene chain that function as a chromophore are responsible for the yellow, orange, and red colours of carotenoids [15][16]. There are two types of carotenoids present in carrot; namely, carotenes and xanthophylls. The major carotenoids (Figure 3A) in carrot roots are β-carotene (75%); α-carotene (23%); lutein (1.9%); and β-cryptoxanthin, lycopene, and zeaxanthin [17].
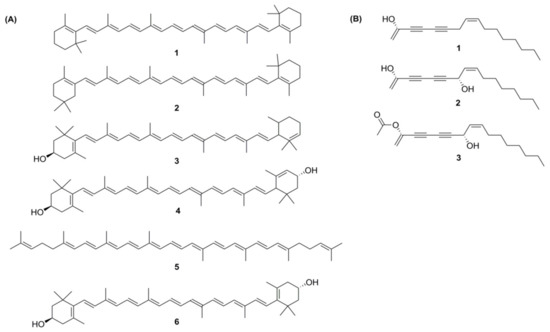
Figure 3. (A) Structures of carotenoids: (1) α-carotene; (2) β-carotene; (3) β-cryptoxanthin; (4) lutein; (5) lycopene; (6) zeaxanthin; (B) Structures of the polyacetylenes: (1) falcarinol; (2) falcarindiol; (3) falcarindiol-3-acetate.
5. Polyacetylenes
Polyacetylenes are a prominent group of non-volatile bioactive phytochemicals that comprise at least two conjugated triple C–C bonds. Plants of the Apiaceae family (to which the carrot belongs) contain aliphatic C17-polyacetylenes of the falcarinol type [18]. Recent studies on the biological activity of polyacetylenes have indicated their potential to improve human health due to anticancerous, antifungal, antibacterial, anti-inflammatory, and serotogenic effects. These findings suggest targeting vegetables with elevated levels of bisacetylenic oxylipins, such as falcarinol; and due to the abundant availability, high diversity of cultivars, worldwide experience, and high agricultural yields, carrot (Daucus carota L.) genotypes are a very promising target vegetable [3].
From more than 1400 polyacetylenes identified in plants, 12 polyacetylenes were isolated from carrot. Out of the twelve, falcarinol, falcarindiol, and falcarindiol-3-acetate are essential polyacetylenes predominately found in carrot roots (Figure 3B). The other nine polyacetylenes that are isolated from carrot are: (E)-isofalcarinolone, falcarindiol-8-acetate, 1,2-dihydrofalcarindiol-3-acetate, (E)-falcarindiolone-8-acetate, (E)-falcarindiolone-9-acetate, 1,2-dihydrofalcarindiol, (E)-1-methoxy-falcarindiolone-8-acetate, (E)-1-methoxy-falcarindiolone-9-acetate, and panaxydiol [3][19].
6. Ascorbic Acid
l-ascorbic acid or vitamin C (Figure 4) is one of the most abundant water-soluble low molecular weight antioxidants found throughout the kingdom Plantae. It is known to play a central role in regulating the cellular redox potential in cells [20][21]. As humans and some other primates lack the ability to synthesize and store vitamin C, they depend on fresh fruits and vegetables to cover their daily requirements (75–90 mg RDA). All recent studies point toward a diet rich in vitamin C for improving human health. Troesch et al. [22], suggest that vitamin C should be a clear target for the nutritional enhancement of horticultural crops. The accumulation of vitamin C within the same species may vary between different cultivars [21][23][24], tissue types [25], and developmental stages [24][25]. Regardless of this variability, vitamin C is tightly regulated through net biosynthesis, recycling, degradation/oxidation, and/or intercellular and intracellular transport.
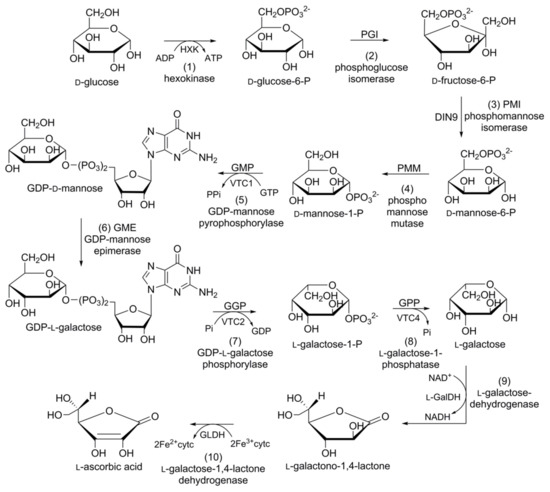
Figure 4. Schematic representation of the biosynthetic pathway of ascorbic acid in carrot: (1) hexokinase; (2) phosphoglucose isomerase; (3) phosphomannose isomerase; (4) phosphomannose mutase; (5) guanosine diphosphate (GDP)-mannose pyrophosphorylase; (6) GDP-mannose epimerase; (7) GDP-l-galactose phosphorylase; (8) l-galactose-phosphatase; (9) l-galactose-dehydrogenase; (10) l-galactose-1,4-lactone dehydrogenase.
References
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre-and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506.
- Food and Agriculture Organization of the United Nations Carrots and Turnips. Available online: (accessed on 10 July 2019).
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C 17-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222.
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant. Foods Hum. Nutr. 2013, 68, 163–170.
- Umar, G.; Kaur, S.; Gurumayum, S.; Rasane, P. Effect of Hot Water Blanching Time and Drying Temperature on the Thin Layer Drying Kinetics of and Anthocyanin Degradation in Black Carrot (Daucus carota L.) Shreds. Food Technol. Biotechnol. 2015, 53, 324–330.
- Nguyen, H.H.V.; Nguyen, L.T. Carrot processing. In Handbook of Vegetable Preservation Processing, 2nd ed.; Hui, Y.H., Evranuz, E.Ö., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 449–478.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901.
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2013, 97, 574–581.
- Czepa, A.; Hofmann, T. Quantitative Studies and Sensory Analyses on the Influence of Cultivar, Spatial Tissue Distribution, and Industrial Processing on the Bitter Off-Taste of Carrots (Daucus carota L.) and Carrot Products. J. Agric. Food Chem. 2004, 52, 4508–4514.
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32.
- Águila Ruiz-Sola, M.; Rodríguez-Concepción, M. Carotenoid biosynthesis in arabidopsis: A colorful pathway. BioOne 2012, 1–28.
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of carotenoids in carrot: An underground story comes to light. Arch. Biochem. Biophys. 2013, 539, 110–116.
- Søltoft, M.; Bysted, A.; Madsen, K.H.; Mark, A.B.; Bügel, S.G.; Nielsen, J.; Knuthsen, P. Effects of organic and conventional growth systems on the content of carotenoids in carrot roots, and on intake and plasma status of carotenoids in humans. J. Sci. Food Agric. 2011, 91, 767–775.
- Christensen, P.L. Aliphatic C17-Polyacetylenes of the Falcarinol Type as Potential Health Promoting Compounds in Food Plants of the Apiaceae Family. Rec. Patents Food Nutr. Agric. 2011, 3, 64–77.
- Schmiech, L.; Carole, A.; Witulski, B.; Hofmann, T. Structure determination of bisacetylenic oxylipins in carrots (Daucus carota L.) and enantioselective synthesis of falcarindiol. J. Agric. Food Chem. 2009, 57, 11030–11040.
- Fotopoulos, V.; Kanellis, A.K. Altered apoplastic ascorbate redox state in tobacco plants via ascorbate oxidase overexpression results in delayed dark-induced senescence in detached leaves. Plant. Physiol. Biochem. 2013, 73, 154–160.
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as seen through plant evolution: The rise of a successful molecule? J. Exp. Bot. 2013, 64, 33–53.
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698.
- Mellidou, I.; Chagné, D.; Laing, W.A.; Keulemans, J.; Davey, M.W. Allelic variation in paralogs of GDP-L-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant. Physiol. 2012.
- Mellidou, I.; Keulemans, J.; Kanellis, A.K.; Davey, M.W. Regulation of fruit ascorbic acid concentrations during ripening in high and low vitamin C tomato cultivars. BMC Plant. Biol. 2012, 12, 239.
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schünemann, N.; Wright, M.; MacRae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J. Exp. Bot. 2009, 60, 765–778.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Revisions:
2 times
(View History)
Update Date:
07 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




