
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel Mota-Rojas | + 3999 word(s) | 3999 | 2021-06-15 11:31:28 | | | |
| 2 | Nora Tang | + 924 word(s) | 4923 | 2021-07-01 04:02:43 | | | | |
| 3 | Catherine Yang | Meta information modification | 4923 | 2021-09-28 11:10:57 | | |
Video Upload Options
Behavioral thermoregulation, in contrast, depends on voluntary decisions. Like what occurs with the physiological mechanisms, thermal stimuli are detected by the afferent pathway that transfers the message to the spinal cord and cerebral cortex, influencing the level of perceived thermal comfort and the individual’s decision to gain or lose heat. These thermoregulating behaviors entail goal-oriented actions learned through reinforcement, as was demonstrated long ago.
1. Introduction
Living beings have developed various adaptive mechanisms for the many alterations their environment may undergo depending on the place, time, or season in question. These mechanisms include morphological, physiological, and behavioral changes that allow them to confront variable conditions and, in this way, regulate their physiological capacities [1][2][3][4].
Examples could include the long, highly-vascularized ears ofOryctolagus cuniculusfor dissipating heat, the development of thermogenesis from brown adipose tissue (BAT) in placental (eutherian) mammals, the reduced thickness of subcutaneous fat in ruminants from arid regions [5], and the huddling of seals as temperatures decrease [6][7][8]. Physiological mechanisms consist of involuntary effectors that produce mostly automatic responses that generate or dissipate heat upon the activation of thermoreceptors and the arrival of information to the hypothalamus, relayed through the spinal cord and midbrain. It is essential to understand that the principle thermo-effector tissues include the cutaneous blood vessels, since (i) many metabolic sources of heat (liver, heart, limb muscles) are distant from the skin, where heat is lost; and (ii) body tissues are poor conductors [9]. Some species have specialized thermoregulating organs, like the rat’s tail, that dissipate heat quickly due to its large surface area and dense vascularization
Behavioral thermoregulation, in contrast, depends on voluntary decisions. Like what occurs with the physiological mechanisms, thermal stimuli are detected by the afferent pathway that transfers the message to the spinal cord and cerebral cortex, influencing the level of perceived thermal comfort and the individual’s decision to gain or lose heat. One of the most basic thermoregulating behaviors consists of searching out cold or hot habitats that allow the organism to alter its rate of heat loss or gain. In contrast, the most complex thermoregulating behaviors include making nests or burrows [6], social behaviors like huddling with conspecifics [10], and human behaviors like wearing clothes or turning on an air conditioner [7].
In mammals, conserving a relatively constant core body temperature (37 °C in most species) has been essential in maintaining an optimal body system functionality and the sensitive chemical and physical processes involved. Temperatures above 45 °C can cause fatal brain injuries, while those below 27–29 °C can cause cardiac fibrillation, a progressive decrease in respiratory rate, and even death [1][9].
Scientific knowledge indicates that controlling the body’s core temperature is essential for survival [11][12]. Under conditions where thermoregulating mechanisms cannot re-establish body temperature, individuals suffer thermal stress [13]. Observation of the offspring of meerkats (Suricata suricatta), for example, a species that inhabits arid regions, has revealed reduced growth and survival rates when ambient temperatures are high [14].
In areas that produce food of animal origin for human consumption, temperature also plays an essential role because poultry, swine, and cattle are all particularly vulnerable due to their high metabolic and growth rates and elevated production levels. Stress caused by intense heat before slaughtering [15], for instance, stimulates muscle glycogenolysis that results in pale, soft exudative meat (PSE) characterized by a low water-holding capacity (WHC). In contrast, stress due to chronic heat reduces the animal’s muscle glycogen reserves, resulting in dark, firm, and dry (DFD) meat with high pH There are also reports on production rates due to the effects of temperature, as in the case of the water buffalo (Bubalus bubalis), which, during periods of high ambient temperatures, have been shown to reduce their milk production, growth, and fertility rates due to an inhibition effect on the enzymatic activity generated by extreme heat [16][17][18][19][20][21][22][23].
Infrared thermography is a technique used in both veterinary and human medicine to quantify the skin’s surface temperature based on visualizations of thermographic changes [18][20]. Since thermal stress affects the welfare and productivity of animals [19][20][23][24][25][26][27], studies designed to achieve a complete understanding of thermoregulating mechanisms using tools like infrared thermography (IRT) [17][18][19][20][21][27][28][29] would allow us to comprehend the effects that factors like climate change has on different species.
Thermography can detect the release of surface heat in the form of thermographic images. Thus IRT detects the peripheral microcirculation controlled by the autonomous nervous system, whose parasympathetic element causes the peripheral vasoconstriction of the capillaries nearest the skin, produced by the neurosecretion of catecholamines [28][29]. At the same time, affecting the temperature in situations that are stressful for animals [20] are factors such as post-birth hypothermia [30][31][32], weaning [33] before slaughter [34][27][35], or such surgical procedures as castration [36], and the effect of necessary pre-operatory procedures, including anesthesia [29].
Negative handling in farm animals (shouting and hitting) leads to poor animal welfare, more fear, acute and chronic stress, fear reactions being the most immediate responses that animals show to potentially dangerous stimuli in their environment [37][38][39]. Having mentioned the potential scope and implications of neurophysiological and behavioral studies of thermal responses, the data obtained in scientific studies will help propose solutions to problems related to thermal stress, fear, and other situations that threaten the health and welfare of domestic and wild animals [19][20][21][22][40][41][42][43]. This review, therefore, analyzes the main anatomical structures and neural pathways that allow the generation of autonomous and behavioral mechanisms that regulate body heat in mammals, information that can be used in related disciplines and areas of opportunity.
2. Neurophysiological Responses for Controlling Hyperthermia
Under normal resting conditions, the body generates and dissipates heat to maintain temperature stability. A human body temperature above 104 °F (40 °C) is defined as severe hyperthermia; that is, a state of high temperature that, in normothermic organisms, triggers mechanisms as cutaneous vasodilation and eccrine sweating or evaporative cooling (conduction and convection), which constitute the principal means of modulating such alterations [44]. Body heat is significantly regulated by the central nervous system (CNS), which receives, deciphers, and sends signals through diverse structures to activate the mechanisms that manage heat dissipation. In some studies, the POA has been stimulated directly with heat to demonstrate its importance in thermoregulation.
The skin has a complex, sympathetic innervation that includes several nervous structures: vasodilators, vasoconstrictors, sudomotors, pilomotors, sensory fibers, or thermoreceptors [45]. Thanks to its structures, the skin can detect changes in ambient temperature and transmit the perceived stimuli directly to the POA, permitting the generation of effective responses to defend the organism’s thermal homeostasis [46]. In addition, its nervous structures receive nervous stimuli sent by the heat-loss center to activate mechanisms like sweating and vasodilatation [47]. It is important to note that the activation of all these mechanisms is controlled by the release of diverse chemical substances that function as messengers inside the communication network called the nervous system [45].
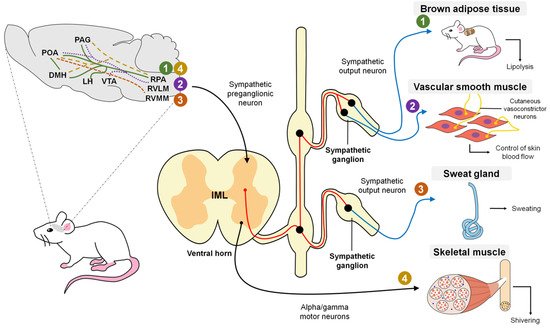
In a general description, the POA contains warm-sensitive neurons that, upon perceiving an alteration, send a stimulus to the sympathetic preganglionic neurons distributed in the nucleus of the intermediolateral area of the spinal cord, which connects to the sympathetic ganglia responsible for innervating the blood vessels, cutaneous vessels and diverse glandular structures that participate in modulating hyperthermia [1]. Similarly, the information perceived by the cutaneous thermoreceptors is transmitted through synapses to the dorsal horn and trigeminal nerve, and from there is relayed to the lateral parabrachial nucleus, which connects to various nervous regions connected to the POA [1].
Nowadays, the mechanism that allows the integration of the information captured by the cutaneous thermoreceptors remains unknown. As mentioned above, organisms use two main mechanisms to control hyperthermia: cutaneous vasodilatation and evaporative cooling.
The function of cutaneous vasodilatation is to divert central circulation into peripheral circulation to dissipate heat using blood as a vehicle. Nowadays, two mechanisms of vasodilatation are known: active vasodilation, characterized by augmenting blood flow by increasing nervous activity, and passive vasodilation, which also increases blood flow, but by reducing the activity of the vasoconstrictor nerve [45][48] (Figure 1). Some studies speculate on control through a network of active neurogenic vasodilators in specific sites of the glabrous skin [49]. As with the mechanisms for controlling hypothermia, the rostral raphe pallidus and rostral ventrolateral medulla (RVLM) participate in inducing vasodilatation.
Regarding vasodilatation, it is believed that the dorsomedial hypothalamic nucleus (DHM) is not required for cooling since the probability of direct projections between the POA and rostral raphe pallidus (rRPA) has already been mentioned [50] even though the ventral tegmental area (VTA) and rostroventrolateral PAG have been posited as brain regions that substitute the chemical stimulus to the rRPA from the DHM [51] (Figure 2).
It is not clear whether, as in hypothermia, the population of glutamatergic cells is responsible for inducing heat-dissipating mechanisms since some studies have described the participation of GABAergic cells [52]. However, the participation of any of these cell populations is possible as evidence indicates that a large portion of the POA neurons express for these two receptors. In addition, since these two populations are heterogeneous, it is necessary to identify objective molecular targets that will enable better understanding of the efferent pathways that activate the associated mechanisms [7].
In non-human mammals, other mechanisms may cool by evaporation with species-specific variations; examples include salivation and spreading saliva over the entire cutaneous surface or fur and panting performed through the respiratory tract [46]. Results showed that sweating activity increased by 80% in the hyperthermal state, reflecting its predominance in that condition; in other words, evaporative cooling constituted the main mechanism of heat dissipation [53]. This process occurs through the eccrine glands, which consist of a spiral secretor in the form of a spiral-shaped, tubular gland that contains a secreting and a proximal conductor located on the dermis, where multiple nervous fibers are enveloped by a dense network of capillaries are found [54]. Accompanied by a group of other cell types, these fibers respond to hydrostatic pressure by generating sweat [55].
It has been determined that sweating in rodents is mediated by the release of acetylcholine, a chemical mediator in the synaptic areas of the sympathetic nerves located in the peripheral sweat glands [7]. Specifically, sympathetic innervation is stimulated by a package of nerves that terminates in the preganglionic neurons residing in the intermediolateral cells of the spinal cord.
The column of IML cells has projections to the rostral ventromedial medulla (RVMM), which correlates to sweating in cats and humans [56]. Currently, the stimulation pathways between the RVMM and POA are not entirely known, so they are still under study [7] (Figure 2).
An important finding is that the mechanism of evaporation demands a high caloric cost for the organism and entails significant physiological alterations, such as excessive water loss that alters osmotic stability. An option is high caloric density diets so that the individual can replenish energy losses with a small amount of food. Nonetheless, in species that inhabit arid regions during seasons marked by a drastic shortage of food and water, there are reports of adaptations that allow a considerable temperature increase that may induce a state of hyperthermia which reduces energy costs and water loss by up to 50%. This adaptive process may benefit from the recent substantial increase in ambient temperatures worldwide [57].
Another alteration caused by states of acute hyperthermia is hypoxia, which affects mainly highly irrigated organs like the intestinal mucosa, producing tissue damage that results in increased permeability [58]. For this reason, intensive production systems have been forced to design methods to reduce the impact of heat stress on their animals [59].
Although the cooling mechanisms of vasodilatation and evaporation are described in the literature individually, and it is believed that distinct nervous circuits mediate them, they are broadly related because vasodilatation provides heat and blood plasma; that is, the resources used as the fluid required for evaporation to occur through sweating, salivation, and panting [44]. Studies have also documented that when sweat and salivary glands are activated, they release an enzyme that catalyzes bradykinin, a peptide with a high capacity for stimulating vasodilatation. When released into the interstitial space, bradykinin causes active vasodilatation, suggesting that it is not a mediator of the activation of cutaneous vasodilatation [60].
During dynamic exercise, the organism concentrates large quantities of metabolic heat produced by muscular contractions. The body’s deep temperature rises due to the heat generated, so cooling mechanisms like vasodilation and evaporation are activated to dissipate excess heat [61]. These specific mechanisms are considered independent from the changes in deep temperature at the onset of exercise; in other words, sweating and vasodilatation can be mediated by exercise even before the organism registers changes in its deep temperature [62]. It has been observed that the fatigue generated by exercise works as an inhibitory stimulus sent to the hypothalamus in response to hyperthermia, where it causes a decrease in the resistance to exercise that functions as a heat-dissipating mechanism [63].
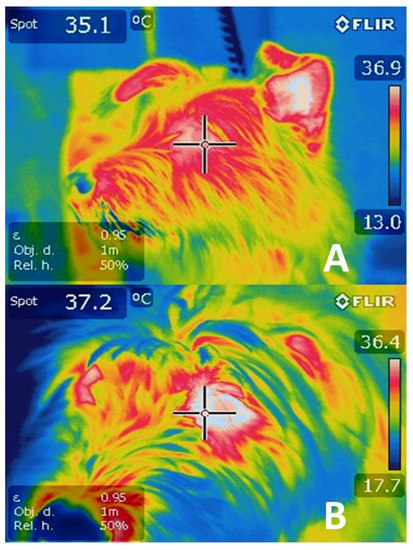
Tanda [64] carried out a study with runners utilizing IRT that allowed him to achieve his main objective: to relate the concentration of metabolic heat with ambient temperature, genetic traits, training, acclimatization, and skin surface temperature. Depending on the capacity of different bodies to dissipate heat through the methods outlined previously, the runners presented central temperatures from 38.5 to 40 °C at the end of the competitions, whether the distances involved were short or long. The study concluded that skin temperature represents the main variable that controls heat exchange during body-environment interaction and that this is due to changes in blood flow caused by vasoconstriction and vasodilatation in the skin. To appreciate changes in ocular temperature registered by IRT in dogs subjected to exercise, see Figure 3.
Today, it is of great clinical importance to monitor the body temperature of certain mammal species involved in sporting activities [65]. Methods have been created that allow the objective measurement of temperature changes on the body’s surface, including the aforementioned infrared thermography (IRT) technique [28]. A pilot study using IRT was conducted in 2017 to demonstrate the changes observed in horses subjected to different times and intensities of physical exercise. All the regions of interest showed an average temperature increase of 2 °C, indicating that IRT is an effective tool, though additional research is required to improve its performance [66].
The fear response is another phenomenon that triggers behavioral and physiological changes through which organisms seek to increase their chances of survival by reacting adequately to threats occurring in the environment [67]. It is not clear how the nervous system participates in the fear response, but it has been suggested that the onset of these reactions and the changes observed as a consequence depend on connections among the thalamus, amygdala, and hypothalamus. It is important to recall that the DHM contains nerve cells to trigger thermogenesis and cutaneous vasoconstriction [68]. It is well-known that the amygdala is a region of neural control involved in the emotions but also plays a vital role in fear conditioning, and it has been noted that it has synaptic connections that endow it with the ability to store memories related to threat stimuli or fear conditioning [69].
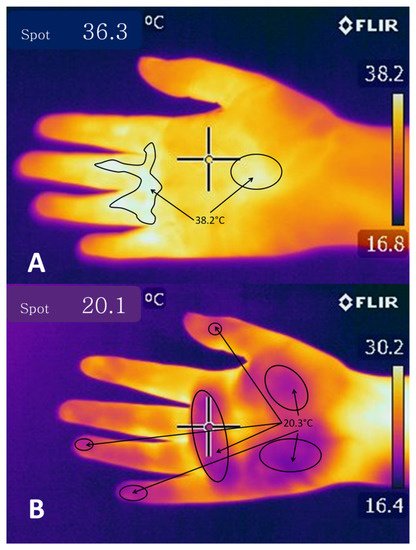
Research has shown that when threats trigger fear reactions in rats, these animals undergo various cardiovascular changes involving a decrease in blood perfusion in areas like the tail and limbs, which are considered zones that have a greater capacity to dissipate heat and that in threatening situations are the most exposed parts of the body. The decreased perfusion is due to cutaneous vasoconstriction of these specific areas, perhaps because the rat is preparing for either “fight-or-flight” and so acts to prevent significant blood loss through these vulnerable anatomical structures [67][70]. This response has been observed under IRT as a concentration of large amounts of heat on the dorsal surface of the rats’ body, and shallow temperatures in the limbs and skin surface since this redistribution of the blood also modifies heat concentrations [70]. As a result, recent findings indicate that behavioral, physiological, and emotional modifications associated with stressful stimuli can be measured not only by utilizing such biomarkers as lactate, glucose, and cortisol, among others [71][72], but also by methods like infrared thermography [19][20][21], observations of body language and facial expressions [38][73], and more advanced techniques, such as electroencephalography (EEG) and computed tomography (CT) [19].
3. Neurophysiological Responses for Controlling Hypothermia
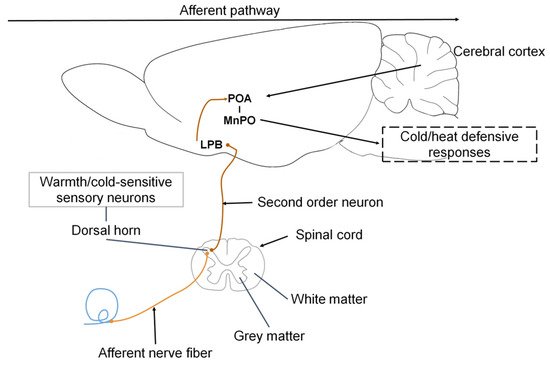
Ascending signals from the thermoreceptors in the skin, viscera, and spinal cord reach the cold-sensitive sensory neurons innervating the superficial laminae of the dorsal horn, which then send glutamatergic projections to the lateral parabrachial nucleus (LPB), specifically the external lateral LPB (LPBel) [7]. The cold-activated LPB neurons send glutamatergic projections to the midline POA, specifically the median preoptic nucleus (MnPO), which, through efferent pathways involving the sympathetic and somatic motor systems, trigger cold-defensive responses, like vasoconstriction and thermogenesis to block heat loss [1].
Madden and Morrison [74] mention that the thermogenic efferent pathway (Figure 4) involves an inhibitory output of the POA that affects the hypothalamic neurons in the dorsomedial hypothalamus. The thermogenesis-promoting neurons of the dorsomedial hypothalamic nucleus (DMH) activate premotor neurons in the raphe pallidus area (RPa) that, in turn, send a descending excitatory drive to the spinal neurons (sympathetic preganglionic neurons for BAT; motor neurons for shivering). The difference lies in the fact that no relay in the DMH is required because the inhibitory output of the POA directly impacts the sympathetic premotor cutaneous vasoconstrictor neurons in the raphe.
In response to cold, the sympathetic nerve fibers that innervate the cutaneous vasculature are activated, causing cutaneous vasoconstriction that (i) reduces heat transfer with the environment and (ii) conserves heat in the center of the organism’s body [74] Those authors observed that inhibiting the MnPO neurons by microinjections of GABA caused vasoconstriction in the tails of mice (Mus musculus) due to the increased activity of the sympathetic CVC nerve fibers. The above suggests that an output from the MnPO inhibits the sympathetic CVC nerve fibers. Excitation of the rostral raphe pallidus (rRPA) also increases vasoconstriction and reduces the cutaneous temperature of the tail (Figure 5), while its inhibition blocks vasoconstriction [7].
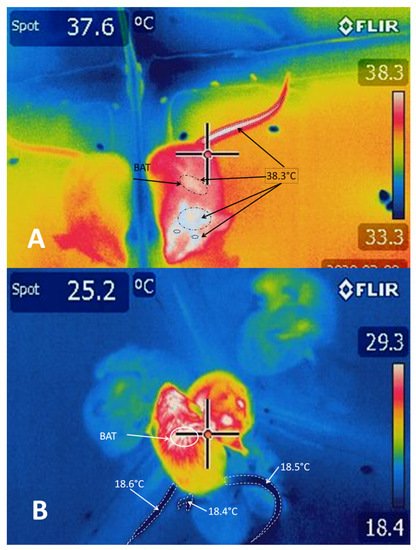
Although the raphe pallidus area (RPa) has sympathetic premotor neurons for cutaneous vasoconstriction [77], it appears that the inhibitory output from the MnPO is an indirect input to the sympathetic CVC premotor neurons in the RPa [78][79]. The skin cooling activates neurons in the POA to directly excite the RPa’s CVC premotor neurons by activating glutamatergic receptors [80]. In response, the RPa’s CVC premotor neurons trigger cutaneous vasoconstriction through the excitatory glutamatergic and serotoninergic projections to the preganglionic neurons in the intermediolateral cell column of the spinal cord [74] However, the RVLM plays only a minor role in CVC activity compared to the RPa’s sympathetic premotor neurons.
Brown adipose tissue (BAT) is a specialized organ for rapid heat production. Tan and Knight [7] affirm that the release of norepinephrine from this sympathetic innervation induces a mitochondrial leak in BAT that consists of a facilitated proton leak through mitochondrial membranes of the brown adipocytes that occurs due to the elevated release of the uncoupling protein-1 (UCP1) This process is known as non-shivering or BAT thermogenesis. [81] and dorsomedial hypothalamic nucleus (DMH) [82], since chemical stimulation in these regions induces BAT thermogenesis.
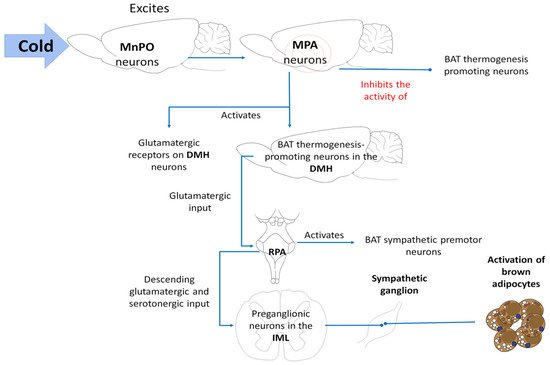
In cold environments, the cool-afferent input to the median preoptic nucleus (MnPO) excites its neurons, which in response inhibit the activity of a population of inhibiting neurons in the medial preoptic area (MPA), suppressing activation of the BAT thermogenesis-promoting neurons [74]. In response to both cold stimuli and fever, activation of the BAT thermogenesis-promoting neurons in the DMH is likely due to the elimination of the active thermogenesis-suppressing output and activation of glutamatergic receptors in the DMH neurons [83]. However, activation of a subpopulation of neurons that contain leptin receptors (LepR) has been detected in mice [84][85]. These glutamatergic neuropsins in the POA can also be expressed in the retina, skin, adrenal glands, liver, heart, and pancreas [86].
Activation of the BAT sympathetic premotor neurons in the RPa, likely through glutamatergic input from the DMH to the RPa [87][88], promotes the descending glutamatergic and serotonergic input to the spinal cord, since glutamate and serotonin in the spinal cord contribute to activating BAT [74] (Figure 6).
Tan and Knight [7] noted that the regulation of shivering also involves a group of structures that govern other physiological responses. Observations from decades ago showed that direct cooling of the POA fostered shivering induced by environmental cold in goats Subsequently, those neurons activate the somatic premotor neurons for muscle shivering found in the RPa. Contrary to what was observed in the neural circuit for the sympathetic control of BAT, where the RPa neurons activate sympathetic preganglionic neurons in the intermediolateral cell column (IML), in this case, the descending input from the RPa for shivering activates alpha and gamma motor neurons [89] in the ventral horn of the spinal cord of rats (Figure 7).
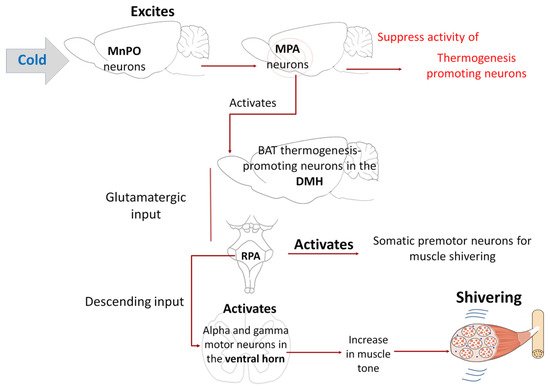
Although various studies have demonstrated the existence of direct descending projections from RPa neurons towards ventral horn somatomotor neurons, the pathways and mechanisms through which the alpha/gamma motor neurons are activated during shivering are not yet fully understood [7][74].
4. Thermoregulating Behavior in Mammals
Animals are also capable of performing voluntary behaviors that alter their local thermal environment. The above suggests that thermoregulating behaviors are driven by the same motivational system that preserves other behaviors—eating and drinking, for example—that arise in response to homeostatic needs [7]. The former includes strategies to conserve body heat, such as adopting certain postures (ball-like position) or basking under the sun, and nest-building, huddling, and nest-sharing. Mammals perform these behaviors when exposed to severe thermal conditions in which their autonomous regulation loses effectiveness, and valuable body resources like water and energy could be compromised.
Although thermal salivation decreases when lesions occur to the anterior [90], lateral [91], and ventromedial hypothalamus [92], it is believed that the simplest pathway is the one through which the preoptic area of the hypothalamus (POA) activates lateral hypothalamic inputs to the superior salivary nucleus [93]. However, grooming activity can be induced by increasing the temperature of the posterior—not anterior—hypothalamus, suggesting that some behavioral thermoregulating responses may be triggered in areas distinct from the POA [94]. Furthermore, the increase of warmth-induced locomotor activity that appears in rats upon stimulating the septal area, ventral midbrain, and dorsal medulla has not been affected after lesions in either these areas or in the POA [95][96]. In contrast, studies with rats have shown that activating the POA with heat induces prone extension behavior [95], while lesions in this region, and the ventral part of the MnPO, reduce or eliminate this behavior [96][97].
The evidence suggests that local cooling or heating of the POA is sufficient but not necessary for activating most thermoregulatory behaviors that reverse changes in the core body temperature [1]. Observations have shown that optogenetic stimulation of POAPACAP/BDNFneurons activated by heat induces cold-seeking but inhibits nest-building [68]. Chemogenetic stimulation of POALepRneurons induces prone extension behavior to dissipate heat in mice [98]. Similarly, autonomous and behavioral responses to manipulations of the POA can stimulate thermoregulating behaviors, likely through the dorsomedial hypothalamic nucleus (DMH) [99].
Several authors mention that lesions that ablate the POA in rats leave most thermoregulating behaviors intact [96][99][100]. Carlisle [100] observed that Sprague-Dawley rats held in a cold environment after lesions to the POA improved their responses to the heat-reinforced operant procedure to which they were subjected. Probably to compensate for their loss of autonomous thermoregulation.
Most lesioning experiments have been unsuccessful in identifying the forebrain region necessary for thermoregulating behaviors, as the POA is for autonomous responses. Observations of primates have shown that the thalamus plays an important role in temperature perception [101][102]. However, recent studies with rodents have demonstrated that even after this area is injured, no alterations are detected in behaviors related to thermoregulation [75].
The failure to block thermoregulating behaviors by injuring the POA has led some researchers to conclude that this area is not involved in those specific responses [103]. According to Tan and Knight [7], these conflicting results could be explained by the complexity of the POA circuit, which contains many different intermingled cell types that make it difficult to interpret the results of lesioning experiments lacking adequate cell-type specificity. When not specific, those injuries cause hyperphagia and obesity in rats, suggesting that the ARC functions as an anxiety center [104], while ablation of a specific type of ARC cells (AgRP neurons) causes starvation in mice [105]. Thus, we hope that future research will focus on manipulations of specific cell types to re-analyze the role of the POA and downstream structures in controlling thermoregulating behaviors.
References
- Morrison, S.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308.
- Nakamura, K.; Morrison, S.F. Central efferent pathways for cold-defensive and febrile shivering. J. Physiol. 2011, 589, 3641–3658.
- Guo, Y.-Y.; Hao, S.; Zhang, M.; Zhang, X.; Wang, D. Aquaporins, evaporative water loss and thermoregulation in heat-acclimated Mongolian gerbils (Meriones unguiculatus). J. Therm. Biol. 2020, 91, 102641.
- Wen, J.; Bo, T.; Zhang, X.; Wang, Z.; Wang, D. Thermo-TRPs and gut microbiota are involved in thermogenesis and energy metabolism during low temperature exposure of obese mice. J. Exp. Biol. 2020, 223, 218974.
- Ward, D. The Biology of Desert; Oxford University Press: New York, NY, USA, 2009.
- Terrien, J. Behavioral thermoregulation in mammals: A review. Front. Biosci. 2011, 16, 1428–1444.
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48.
- Legendre, L.J.; Davesne, D. The evolution of mechanisms involved in vertebrate endothermy. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190136.
- Klein, B.G. Cunningham: Fisiología Veterinaria, 5th ed.; Elsevier: Madrid, Spain, 2013.
- Batchelder, P.; Kinney, R.O.; Demlow, L.; Lynch, C.B. Effects of temperature and social interactions on huddling behavior in Mus musculus. Physiol. Behav. 1983, 31, 97–102.
- Fuller-Jackson, J.-P.; Clarke, I.J.; Henry, B.A. Chapter 12: Animal Models for Manipulation of Thermogenesis. In Animal Models for the Study of Human Disease; Conn, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 281–312.
- Wang, T.A.; Teo, C.F.; Åkerblom, M.; Chen, C.; Fontaine, M.T.-L.; Greiner, V.J.; Diaz, A.; McManus, M.T.; Jan, Y.N.; Jan, L.Y. Thermoregulation via Temperature-Dependent PGD2 Production in Mouse Preoptic Area. Neuron 2019, 103, 309–322.e7.
- Gu, Z.; Yang, S.; Leng, J.; Xu, S.; Tang, S.; Liu, C.; Gao, Y.; Mao, H. Impacts of shade on physiological and behavioural pattern of Dehong buffalo calves under high temperature. Appl. Anim. Behav. Sci. 2016, 177, 1–5.
- Van De Ven, T.M.F.N.; Fuller, A.; Clutton-Brock, T.H. Effects of climate change on pup growth and survival in a cooperative mammal, the meerkat. Funct. Ecol. 2020, 34, 194–202.
- Becerril-Herrera, M.; Alonso-Spilsbury, M.; Lemus-Flores, C.; Guerrero-Legarreta, I.; Olmos-Hernández, A.; Ramírez-Necoechea, R.; Mota-Rojas, D. CO2 stunning may compromise swine welfare compared with electrical stunning. Meat Sci. 2009, 81, 233–237.
- Marai, I.; Haeeb, A. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109.
- Mota-Rojas, D.; Miranda-Cortés, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato-Funes, L.; Hernández-Ávalos, I. Neurobiología y modulación de la hipertermia inducida por estrés agudo y fiebre en los animales. Abanico Veter. 2021, 11, 1–17.
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 2021, 70, 1–12.
- Mota-Rojas, D.; Ghezzi, M.D.; Napolitano, F.; Rosmini, M.R.; Guerrero-Legarreta, I.; Martínez-Burnes, J.; Lezama-García, K.; Miranda-Cortés, A.; de la Vega, L.T.; Mora-Medina, P.; et al. Quality of death in the river buffalo (Bubalus bubalis). J. Anim. Behav. Biometeorol. 2021, 9, 1–10.
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal biology in river buffalo in the humid tropics: Neurophysiological and behavioral responses assessed by infrared thermography. J. Anim. Behav. Biometeorol. 2021, 9, 1–12.
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific findings related to changes in vascular microcirculation using infrared thermography in the river buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297.
- Bertoni, A.; Napolitano, F.; Mota-Rojas, D.; Sabia, E.; Álvarez-Macías, A.; Mora-Medina, P.; Morales-Canela, A.; Berdugo-Gutiérrez, J.; Legarreta, I.G.-; Agrarie, F.S.D.S. Similarities and Differences between River Buffaloes and Cattle: Health, Physiological, Behavioral and Productivity Aspects. J. Buffalo Sci. 2020, 9, 92–109.
- Lendez, P.A.; Cuesta, L.M.; Farias, M.V.N.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Alterations in TNF-α and its receptors expression in cows undergoing heat stress. Veter. Immunol. Immunopathol. 2021, 235, 110232.
- Gonçalves, A.A.; Garcia, A.R.; Filho, S.T.R.; da Silva, J.A.R.; de Melo, D.N.; Guimarães, T.C.; Tavares, H.R.; Silva, T.V.G.; de Souza, E.B.; Santos, S.D.S.D.; et al. Scrotal thermoregulation and sequential sperm abnormalities in buffalo bulls (Bubalus bubalis) under short-term heat stress. J. Therm. Biol. 2021, 96, 102842.
- Sevegnani, K.B.; Fernandes, D.P.B.; Da Silva, S.H.M.-G. Evaluation of thermorregulatory capacity of dairy buffaloes using infrared thermography. Engenharia Agrícola 2016, 36, 1–12.
- Lendez, P.A.; Farias, M.V.N.; Cuesta, L.M.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Heat Stress: Its Effect on the Immune Status of Dairy Cows. Rev. Medica Vet. 2020, 101, 7–13.
- Flores-Peinado, S.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Mora-Medina, P.; Cruz-Monterrosa, R.; Gómez-Prado, J.; Hernández, M.G.; Cruz-Playas, J.; Martínez-Burnes, J. Physiological responses of pigs to preslaughter handling: Infrared and thermal imaging applications. Int. J. Veter. Sci. Med. 2020, 8, 71–84.
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98.
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664.
- Caldara, F.R.; Dos Santos, L.S.; Machado, S.T.; Moi, M.; Nääs, I.D.A.; Foppa, L.; Garcia, R.G.; Santos, R.D.K.S.D. Piglets’ Surface Temperature Change at Different Weights at Birth. Asian Australas. J. Anim. Sci. 2014, 27, 431–438.
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal homeostasis in the newborn puppy: Behavioral and physiological responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–12.
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2021, 9, 1–10.
- Yáñez, P.A.; Mota-Rojas, D.; Ramírez-Necoechea, R.; Castillo-Rivera, M.; Roldan, P.; Mora-Medina, P.; González, M. Application of infrared thermography to assess the effect of different types of environmental enrichment on the ocular, auricular pavilion and nose area temperatures of weaned piglets. Comput. Electron. Agric. 2019, 156, 33–42.
- Mota-Rojas, D.; Becerril-Herrera, M.; Roldan-Santiago, P.; Alonso-Spilsbury, M.; Flores-Peinado, S.; Ramírez-Necoechea, R.; Ramírez-Telles, J.; Mora-Medina, P.; Pérez, M.; Molina, E.; et al. Effects of long distance transportation and CO2 stunning on critical blood values in pigs. Meat Sci. 2012, 90, 893–898.
- Weschenfelder, A.V.; Saucier, L.; Maldague, X.; Rocha, L.M.; Schaefer, A.L.; Faucitano, L. Use of infrared ocular thermography to assess physiological conditions of pigs prior to slaughter and predict pork quality variation. Meat Sci. 2013, 95, 616–620.
- Pérez-Pedraza, E.; Mota-Rojas, D.; González-Lozano, M.; Guerrero-Legarreta, I.; Martinez-Burnes, J.; Mora-Medina, P.; Cruz-Monterrosa, R.; Ramírez-Necoechea, R. Infrared Thermography and Metabolic Changes in Castrated Piglets due to the Effects of Age and the Number of Incisions in the Testicles. Am. J. Anim. Veter. Sci. 2018, 13, 104–114.
- Andersen, I.L.; Berg, S.; Bøe, K.E.; Edwards, S. Positive handling in late pregnancy and the consequences for maternal behaviour and production in sows. Appl. Anim. Behav. Sci. 2006, 99, 64–76.
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of Human-Animal Relationship on Animal Productivity and Welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205.
- Mota-Rojas, D.; Napolitano, F.; Strappini, A.; Orihuela, A.; Ghezzi, M.; Hernández-Ávalos, I.; Mora-Medina, P.; Whittaker, A. Pain at the Slaughterhouse in Ruminants with a Focus on the Neurobiology of Sensitisation. Animals 2021, 11, 1085.
- Khongdee, T.; Sripoon, S.; Vajrabukka, C. The effects of high temperature and wallow on physiological responses of swamp buffaloes (Bubalus bubalis) during winter season in Thailand. J. Therm. Biol. 2011, 36, 417–421.
- Narayan, E.; Perakis, A.; Meikle, W. Using Thermal Imaging to Monitor Body Temperature of Koalas (Phascolarctos cinereus) in A Zoo Setting. Animals 2019, 9, 1094.
- Guerrero-Legarreta, I.; Napolitano, F.; Mota-Rojas, D.; Orihuela, A. El Búfalo de Agua en las Américas, Enfoques Prácticos y Experimentales, 2nd ed.; BM Editores: Mexico City, Mexico, 2019; pp. 1–881.
- Napolitano, F.; Mota-Rojas, D.; Guerrero Legarreta, I.; Orihuela, A. The Latin American River Buffalo, Recent Findings, 3rd ed.; BM Editores Press: Mexico City, Mexico, 2020; pp. 1–1558. Available online: (accessed on 14 January 2021).
- Nagashima, K. Central Mechanisms for Thermoregulation in a Hot Environment. Ind. Heal. 2006, 44, 359–367.
- Smith, C.J.; Johnson, J.M. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton. Neurosci. 2016, 196, 25–36.
- Shaun, F.M. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104.
- Kanosue, K.; Crawshaw, L.I.; Nagashima, K.; Yoda, T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 109, 5–11.
- Wu, Y.; Nieuwenhoff, M.; Huygen, F.; van der Helm, F.; Niehof, S.; Schouten, A. Characterizing human skin blood flow regulation in response to different local skin temperature perturbations. Microvasc. Res. 2017, 111, 96–102.
- Hodges, G.J.; Kosiba, W.A.; Zhao, K.; Johnson, J.M. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am. J. Physiol. Circ. Physiol. 2009, 296, H51–H56.
- Tan, C.-H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nat. Cell Biol. 2016, 536, 460–463.
- Ootsuka, Y.; Tanaka, M. Control of cutaneous blood flow by central nervous system. Temperature 2015, 2, 392–405.
- Zhao, Z.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047.
- Sugenoya, J.; Iwase, S.; Mano, T.; Sugiyama, Y.; Ogawa, T.; Nishiyama, T.; Nishimura, N.; Kimura, T. Vasodilator component in sympathetic nerve activity destined for the skin of the dorsal foot of mildly heated humans. J. Physiol. 1998, 507, 603–610.
- Kennedy, W.R.; Wendelschafer-Crabb, G.; Brelje, T.C. Innervation and vasculature of human sweat glands: An immunohistochemistry-laser scanning confocal fluorescence microscopy study. J. Neurosci. 1994, 14, 6825–6833.
- Gagnon, D.; Crandall, C.G. Chapter 13: Sweating as a heat loss thermoeffector. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 211–232.
- Shafton, A.D.; McAllen, R.M. Location of cat brain stem neurons that drive sweating. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R804–R809.
- Nilsson, J.-Å.; Molokwu, M.N.; Olsson, O. Body Temperature Regulation in Hot Environments. PLoS ONE 2016, 11, e0161481.
- Kpodo, K.R.; Duttlinger, A.W.; Radcliffe, J.S.; Johnson, J.S. Time course determination of the effects of rapid and gradual cooling after acute hyperthermia on body temperature and intestinal integrity in pigs. J. Therm. Biol. 2020, 87, 102481.
- Baumgard, L.H.; Rhoads, R. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337.
- Kellogg, D.L.; Liu, Y.; McAllister, K.; Friel, C.; Pergola, P.E. Bradykinin does not mediate cutaneous active vasodilation during heat stress in humans. J. Appl. Physiol. 2002, 93, 1215–1221.
- Yanagimoto, S.; Kuwahara, T.; Zhang, Y.; Koga, S.; Inoue, Y.; Kondo, N. Intensity-dependent thermoregulatory responses at the onset of dynamic exercise in mildly heated humans. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R200–R207.
- Shibasaki, M.; Kondo, N.; Crandall, C.G. Evidence for metaboreceptor stimulation of sweating in normothermic and heat-stressed humans. J. Physiol. 2001, 534, 605–611.
- Nybo, L. Hyperthermia and fatigue. J. Appl. Physiol. 2008, 104, 871–878.
- Tanda, G. Skin temperature measurements by infrared thermography during running exercise. Exp. Therm. Fluid Sci. 2016, 71, 103–113.
- Hinchcliff, K.W.; Kaneps, A.J.; Geor, R.J. Equine Sports Medicine and Surgery; Elsevier: London, UK, 2004; pp. 1–1364.
- Soroko, M.; Howell, K.; Dudek, K.; Wilk, I.; Zastrzeżyńska, M.; Janczarek, I. A Pilot Study into the Utility of Dynamic Infrared Thermography for Measuring Body Surface Temperature Changes During Treadmill Exercise in Horses. J. Equine Veter. Sci. 2018, 62, 44–46.
- Angilletta, M.J.; Youngblood, J.P.; Neel, L.K.; VandenBrooks, J.M. The neuroscience of adaptive thermoregulation. Neurosci. Lett. 2019, 692, 127–136.
- Tan, C.L.; Cooke, E.K.; Leib, D.; Lin, Y.-C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons that Control Body Temperature. Cell 2016, 167, 47–59.e15.
- Rodrigues, S.M.; Schafe, G.E.; LeDoux, J.E. Molecular Mechanisms Underlying Emotional Learning and Memory in the Lateral Amygdala. Neuron 2004, 44, 75–91.
- Vianna, D.M.L.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512.
- Hernández-Avalos, I.; Flores-Gasca, E.; Mota-Rojas, D.; Casas-Alvarado, A.; Miranda-Cortés, A.E.; Domínguez-Oliva, A. Neurobiology of anesthetic-surgical stress and induced behavioral changes in dogs and cats: A review. Veter. World 2021, 14, 393–404.
- Larrondo, C.; Orihuela, A.; Strappini, A.; Acosta-Jamett, G.; Mota-Rojas, D.; Gallo, C. Provision of straw and the presence of undocked lambs reduce the behavioural and physiological expressions of pain and stress associated with tail docking in lambs: A preliminary study. Anim. Prod. Sci. 2021, 61, 423.
- Lezama-García, K. Facial expressions and emotions in domestic animals. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2019, 14, 1–12.
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232.
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 2017, 7, 1–10.
- McGowan, N.E.; Scantlebury, D.M.; Bennett, N.C.; Maule, A.G.; Marks, N.J. Thermoregulatory differences in African mole-rat species from disparate habitats: Responses and limitations. J. Therm. Biol. 2020, 88, 102495.
- Blessing, W.W.; Yu, Y.H.; Nalivaiko, E. Raphe pallidus and parapyramidal neurons regulate ear pinna vascular conductance in the rabbit. Neurosci. Lett. 1999, 270, 33–36.
- Nakamura, K.; Matsumura, K.; Kaneko, T.; Kobayashi, S.; Katoh, H.; Negishi, M. The Rostral Raphe Pallidus Nucleus Mediates Pyrogenic Transmission from the Preoptic Area. J. Neurosci. 2002, 22, 4600–4610.
- Rathner, J.A.; Madden, C.J.; Morrison, S.F. Central pathway for spontaneous and prostaglandin E2-evoked cutaneous vasoconstriction. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R343–R354.
- Tanaka, M.; McKinley, M.J.; McAllen, R.M. Preoptic-Raphe Connections for Thermoregulatory Vasomotor Control. J. Neurosci. 2011, 31, 5078–5088.
- Morrison, S.F.; Sved, A.F.; Passerin, A.M. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R290–R297.
- Zaretskaia, M.V.; Zaretsky, D.V.; Shekhar, A.; DiMicco, J.A. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002, 928, 113–125.
- Madden, C.J.; Morrison, S.F. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am. J. Physiol. Integr. Comp. Physiol. 2004, 286, R320–R325.
- Zhang, Y.; Kerman, I.A.; Laque, A.; Nguyen, P.; Faouzi, M.; Louis, G.W.; Jones, J.C.; Rhodes, C.; Münzberg, H. Leptin-Receptor-Expressing Neurons in the Dorsomedial Hypothalamus and Median Preoptic Area Regulate Sympathetic Brown Adipose Tissue Circuits. J. Neurosci. 2011, 31, 1873–1884.
- Dodd, G.T.; Worth, A.A.; Nunn, N.; Korpal, A.K.; Bechtold, D.A.; Allison, M.B.; Myers, M.G.; Statnick, M.A.; Luckman, S.M. The Thermogenic Effect of Leptin Is Dependent on a Distinct Population of Prolactin-Releasing Peptide Neurons in the Dorsomedial Hypothalamus. Cell Metab. 2014, 20, 639–649.
- Zhang, K.X.; D’Souza, S.; Upton, B.A.; Kernodle, S.; Vemaraju, S.; Nayak, G.; Gaitonde, K.D.; Holt, A.L.; Linne, C.D.; Smith, A.N.; et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nat. Cell Biol. 2020, 585, 420–425.
- Kataoka, N.; Hioki, H.; Kaneko, T.; Nakamura, K. Psychological Stress Activates a Dorsomedial Hypothalamus-Medullary Raphe Circuit Driving Brown Adipose Tissue Thermogenesis and Hyperthermia. Cell Metab. 2014, 20, 346–358.
- Machado, N.L.; Abbott, S.B.; Resch, J.M.; Zhu, L.; Arrigoni, E.; Lowell, B.B.; Fuller, P.M.; Fontes, M.A.; Saper, C.B. A Glutamatergic Hypothalamomedullary Circuit Mediates Thermogenesis, but Not Heat Conservation, during Stress-Induced Hyperthermia. Curr. Biol. 2018, 28, 2291–2301.e5.
- Tanaka, M.; Owens, N.C.; Nagashima, K.; Kanosue, K.; McAllen, R.M. Reflex activation of rat fusimotor neurons by body surface cooling, and its dependence on the medullary raphé. J. Physiol. 2006, 572, 569–583.
- Whyte, D.G.; Johnson, A.K. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (av3v) during acute heat stress in the rat. Clin. Exp. Pharmacol. Physiol. 2005, 32, 457–461.
- Stricker, E.M.; Hainsworth, F.R. Evaporative cooling in the rat: Effects of hypothalamic lesions and chorda tympani damage. Can. J. Physiol. Pharmacol. 1970, 48, 11–17.
- Flynn, F.W.; Evey, L.A.; Mitchell, J.C. Heat-induced saliva secretion and thermoregulation in female rats with ventromedial hypothalamic lesions. Physiol. Behav. 1981, 26, 779–782.
- Saper, C.; Loewy, A. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980, 197, 291–317.
- Tanaka, H.; Kanosue, K.; Nakayama, T.; Shen, Z. Grooming, body extension, and vasomotor responses induced by hypothalamic warming at different ambient temperatures in rats. Physiol. Behav. 1986, 38, 145–151.
- Roberts, W.W.; Mooney, R.D. Brain areas controlling thermoregulatory grooming, prone extension, locomotion, and tail vasodilation in rats. J. Comp. Physiol. Psychol. 1974, 86, 470–480.
- Roberts, W.W.; Martin, J.R. Effects of lesions in central thermosensitive areas on thermoregulatory responses in rat. Physiol. Behav. 1977, 19, 503–511.
- Whyte, D.G.; Brennan, T.J.; Johnson, A.K. Thermoregulatory behavior is disrupted in rats with lesions of the anteroventral third ventricular area (AV3V). Physiol. Behav. 2006, 87, 493–499.
- Yu, S.; Qualls-Creekmore, E.; Rezai-Zadeh, K.; Jiang, Y.; Berthoud, H.-R.; Morrison, C.; Derbenev, A.V.; Zsombok, A.; Münzberg, H. Glutamatergic Preoptic Area Neurons That Express Leptin Receptors Drive Temperature-Dependent Body Weight Homeostasis. J. Neurosci. 2016, 36, 5034–5046.
- Almeida, M.C.; Steiner, A.A.; Branco, L.G.S.; Romanovsky, A.A. Neural Substrate of Cold-Seeking Behavior in Endotoxin Shock. PLoS ONE 2006, 1, e1.
- Carlisle, H.J. Effect of preoptic and anterior hypothalamic lesions on behavioral thermoregulation in the cold. J. Comp. Physiol. Psychol. 1969, 69, 391–402.
- Craig, A.D.; Bushnell, M.C.; Zhang, E.-T.; Blomqvist, A. A thalamic nucleus specific for pain and temperature sensation. Nat. Cell Biol. 1994, 372, 770–773.
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666.
- Almeida, M.C.; Vizin, R.C.L.; Carrettiero, D.C. Current understanding on the neurophysiology of behavioral thermoregulation. Temperature 2015, 2, 483–490.
- Choi, S.; Dallman, M.F. Hypothalamic Obesity: Multiple Routes Mediated by Loss of Function in Medial Cell Groups1. Endocrinology 1999, 140, 4081–4088.
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science 2005, 310, 683–685.




