
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lifang Hu | + 7289 word(s) | 7289 | 2021-06-21 11:16:31 | | | |
| 2 | Conner Chen | -2550 word(s) | 4739 | 2021-06-29 11:52:44 | | | | |
| 3 | Conner Chen | -9 word(s) | 4730 | 2021-07-01 04:48:14 | | | | |
| 4 | Conner Chen | -9 word(s) | 4730 | 2021-07-01 04:52:43 | | | | |
| 5 | Conner Chen | Meta information modification | 4730 | 2021-07-01 04:57:09 | | |
Video Upload Options
Mechanotransduction is an important process for living cells and tissues by which they experience and respond to mechanical stimuli. Cellular mechanotransduction is crucial for bone development and physiology, and abnormal cellular mechanotransduction leads to various bone diseases, including osteoporosis (OP) and osteoarthritis (OA). Piezo channels are mechanosensitive ion channels located in the cell membrane and function as key cellular mechanotransducers for converting mechanical stimuli into electrochemical signals. The Piezo channels play crucial roles in numerous physiological and pathological process by functioning as cellular mechanotransducers. Under mechanical stimuli, Piezo channels are opened to make cationic ions cross membrane, which promotes cellular mechanotransduction to adapt to the microenvironment.
1. Piezo Channels
1.1. The Genes, Members, and Structures of Piezo Channels
1.1.1. The Genes and Members of Piezo Channels
Piezo channels have two members in vertebrates: Piezo1 and Piezo2, which have the property of being activated by pressure [1]. In 2010, Coste et al. screened out the ion channel protein (coding gene: Fam38A) that produces the most stable current and the largest response under pressure stimulation [2]. Subsequently, the protein encoded by the Fam38B gene was found through sequence homology [2]. Fam38A and Fam38B are located on human chromosomes 16 and 18, respectively (gene location information was obtained from the NCBI database). Because the Greek word “pίesi” means pressure, Fam38A and Fam38B were named as Piezo1 and Piezo2, respectively [2]. Human Piezo1 and Piezo2 consist of 2521 amino acids and 2752 amino acids, respectively [3]. Mouse Piezo1 and Piezo2 are composed of 2547 amino acids and 2822 amino acids, respectively [4][5] (Table 1).
Table 1. The similarities and differences between Piezo1 and Piezo2.
| Items | Piezo1 | Piezo2 | Reference |
|---|---|---|---|
| Gene | Fam38A | Fam38B | [2] |
| Chromosomal localization | Human chromosome 16 | Human chromosome 18 | NCBI database (https://www.ncbi.nlm.nih.gov/gene/9780 (accessed on 06 June 2021); https://www.ncbi.nlm.nih.gov/gene/63895 (accessed on 06 June 2021)) |
| Amino acid size in humans | 2521 amino acids | 2752 amino acids | [3] |
| Amino acid size in mice | 2547 amino acids | 2822 amino acids | [4][5] |
| Structure | A homotrimer structure resembling a three-bladed propeller | A homotrimer structure resembling a three-bladed propeller | [5][6] |
| Transmembrane pore characteristics | Dilated | Closed | [5] |
| Tissue distribution | Widely distributed in skin, bladder, kidney, lung, endothelial cells, erythrocytes, periodontal ligament cells, trigeminal sensory neurons, dorsal root ganglion, etc. | Trigeminal sensory neurons, dorsal root ganglion, Merkel cells, and somatic neuron cells, etc. | [1][7][8][3][9] |
| Function | Involving in mechanotransduction in a variety of cells | Sensing slight touch and proprioception | [10][11][12][7][8][13][14][15] |
| Activator | Yoda1, Jedi1/2 | Not found yet | [16][17] |
| Inhibitor | Ruthenium red, gadolinium, streptomycin, and GsMTx4 | Ruthenium red, gadolinium, streptomycin, GsMTx4, and FM1-43 | [2][1][18][19][20] |
1.1.2. Structure
Piezo1 and Piezo2 both have a similar homotrimer structure but differ in many aspects. At present, the cryo-electron microscopy structures of mouse Piezo1 and mouse Piezo2 have been obtained [5][6]. However, the structures of human Piezo1 and Piezo2 remain to be resolved. In mouse, Piezo1 is a homotrimer, which is similar to a three-bladed propeller [21]. Piezo1 can be divided into two modules: the peripheral mechanotransduction module and the central ion conduction pore module [21][22] (Figure 1a). The peripheral module consists of the extracellular distal blades, the peripheral helices (PHs), anchors of the transmembrane, and the intracellular beams. The central pore module includes the C-terminal extracellular domains (CEDs), the transmembrane inner helices (IHs) and outer helices (OHs), and the intracellular C-terminal domains (CTDs). The combination of three CEDs forms an extracellular cap [23][24][21] (Figure 1c). The intracellular beam connects the PHs to the CTD [17]. There are many transmembrane helical units (THUs) in the transmembrane region, and THUs are roughly divided into IHs, OHs, and PHs [4] (Figure 1a). The OH is connected to the first peripheral helix near the central axis by a hairpin structure that is formed by four α helices and named the anchor [4]. The C-terminal of each monomer is connected in order as PHs-Anchor-OH-CED-IH-CTD [4][6]. Recently, Geng et al. presented a model of Piezo1′s exquisite plug-and-latch mechanism. More precisely, on the cytoplasmic side, each monomer of Piezo1 has a lateral ion channel: a plug and a latch [25]. Under the pull of the latch, the plug is removed to allow the ions to pass [25]. Zheng et al. found that the intracellular CTDs of Piezo1 can drive the IHs to move by contracting, which rapidly deactivates Piezo1 [26]. These data suggest that the delicate structure of Piezo channels is responsible for its mechanotransduction.
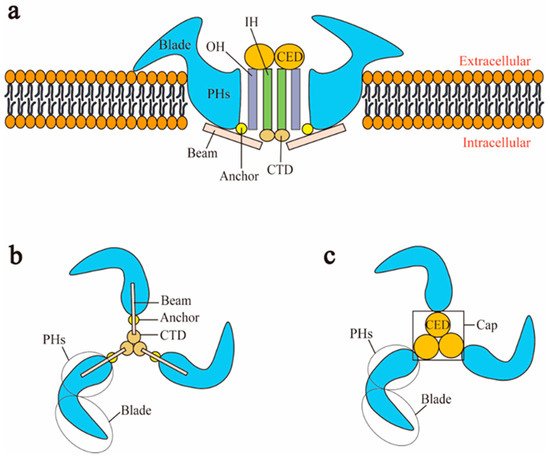
Figure 1. Schematic representation of Piezo1 channel. (a) Lateral view; (b) bottom view; (c) top view. CED: C-terminal extracellular domain; CTD: intracellular C-terminal domain; IH: inner helix; OH: outer helix; PHs: peripheral helices.
Although Piezo2 is roughly similar to Piezo1 in structure, there are many differences in their details. Cryo-electron microscopy structures of Piezo1 and Piezo2 indicated that the central pore of Piezo1 is dilated, while the transmembrane central pore of Piezo2 is closed [5]. The three constriction sites of Piezo2 at L2743, F2754, and E2757 are apparently dilated in the corresponding positions in Piezo1 at L2469, F2480, and G2483, respectively [5]. The ion-permeating properties of Piezo2 are controlled by E2757, which is the only negatively charged residue in the inner helix [5]. In addition, the diameter, depth, and maximum projected area of Piezo2′s intermediate plane openings are 24 nm, 9 nm, and 250 nm2, respectively, while the related parameters of Piezo1 are 18 nm, 9 nm, and 120 nm2, respectively [5].
2. The Role of Piezo Channels in Cellular Mechanotransduction
3. Activators and Inhibitors of Piezo Channels
4. The Role of Piezo Channels in Bone
Bone is a finely mechanosensitive organ and constantly adapts its shape and internal structure to mechanical loads, including body weight, exercise, and gravity [37][38]. Bone homeostasis, maintained by modeling, remodeling, and tissue repair, is regulated by coordinated activities of the bone cells through a process termed cellular mechanotransduction triggered by mechanical stimuli, including fluid shear force, compression, tension, and so on [39][40]. Bone cells include osteoblast lineage (roughly divided into mesenchymal stem cells (MSCs), osteoblasts, and osteocytes) and osteoclasts [41][42]. Osteocytes are terminally differentiated osteoblasts that are derived from MSCs [43][44]. Osteocytes play a vital role in bone homeostasis by regulating the formation and activity of osteoblasts and osteoclasts [44]. In addition, the cartilage that connects the bone in the joints is also an intrinsically mechanosensitive tissue composed of chondrocytes as the only cell type [45]. Chondrocytes, one of the differentiation directions of MSCs, regulate the metabolism of the cartilage extracellular matrix (ECM) to adapt to the mechanical stress environment [46]. At present, many studies have found that Piezo1 is expressed in bone and plays an important mechanotransduction role there [47][34][48][49][50]. Moreover, Piezo channels (Piezo1 and Piezo2) are expressed in chondrocytes and participate in the maintenance of cartilage homeostasis associated with mechanotransduction [51][12][52].
4.1. Piezo1 and Osteocytes
4.2. Piezo1 and Bone Marrow Mesenchymal Stem Cells (BM-MSCs)
Bone marrow mesenchymal stem cells (BM-MSCs) are located in bone marrow and can self-renew [57]. In osteoblastogenesis, mesenchymal stem cells (MSCs) can differentiate into osteoblasts and eventually into osteocytes [58]. Therefore, MSCs play an important role in maintaining normal bone homeostasis [59][60]. Adipogenesis and osteoblastogenesis are two opposite directions of differentiation of mesenchymal stem cells. Adipogenesis-inducible factor inhibits osteoblastogenesis, while osteoblastogenesis-inducible factor blocks adipogenesis [61]. The specific direction of differentiation is precisely regulated by biological, physical, and chemical factors. Physical factors, such as mechanical strain, vibration, and hydrostatic pressure, are important factors in the osteogenesis of MSCs [60]. Sugimoto et al. established a cell culture chamber capable of controlling hydrostatic pressure, in which the increased expression of Piezo1 was detected and the osteogenic differentiation was enhanced in primary MSCs and MSC lines in in vitro research [48]. When MSCs were treated with Yoda1 in vitro, BMP2 (bone morphogenetic protein 2) expression was increased and promoted the differentiation to osteoblasts, which inhibited the differentiation to adipocytes [48]. The results of Sugimoto et al. suggest that mechanical stimulation of hydrostatic pressure induces Piezo1 to promote the differentiation of MSCs into osteoblasts by promoting the expression of BMP2 [48]. Because the above findings of Piezo1 in MSCs are from in vitro studies, further in vivo investigation is needed.
4.3. Piezo1 and Osteoblasts
Osteoblasts are mainly derived from MSCs in the inside and outside of periosteum and the matrix of bone marrow [62]. Mechanical loads associated with body weight, movement, and gravity normally help osteoblasts to build new bone tissue, which ensures that bone grows correctly and remains strong [63][64]. However, mechanical unloading of bone disrupts this process, leading to rapid bone loss [64]. Sun et al. found that Piezo1 is expressed in osteoblasts and helps osteoblasts respond to the mechanical shock of being poked by a microprobe in vitro [49]. Mice with Piezo1 specifically knocked out in osteoblasts failed to grow normally and were stunted in adulthood [49]. Furthermore, data on mice with hindlimb suspension in vivo and osteoblasts with a cell rotation system in vitro suggest that mechanical unloading can inhibit the expression of Piezo1, resulting in dysfunction of osteoblasts and bone formation [49]. Yan et al. silenced Piezo1 with small interfering RNA (siRNA) in the MC3T3-E1 osteoblasts [65]. Subsequent transwell cell migration experiments and cell scratch experiments showed that the number of Piezo1-siRNA cells migrating per well and the rate of scratch healing were significantly reduced, indicating that the Piezo1 gene silencing significantly inhibited the migration ability of MC3T3-E1 osteoblasts [65]. Meanwhile, Yoneda et al. found that Piezo1 activator Yoda1 triggers Ca2+ influx and promotes proliferation in MC3T3-E1 osteoblasts in vitro [66]. In vitro, MC3T3-E1 osteoblasts required Piezo1 to adapt to the external mechanical fluid shear stress, thereby inducing osteoblastic Runx2 (Runt-related transcription factor 2) gene expression, partly through the AKT/GSK-3β/β-catenin pathway [67]. Recently, Wang et al. have found a Piezo1-YAP1-collagen pathway in osteoblasts in vivo and in vitro [47]. More precisely, osteoclast differentiation is regulated by the expression of bone matrix proteins (collagen type II and IX), but these collagens are controlled by Piezo1 in osteoblasts via regulating nuclear translocation of YAP1 [47]. The Piezo1-YAP1-collagen pathway suggests that Piezo1 indirectly regulates bone resorption activity in osteoclasts, thereby affecting bone metabolism.
Interestingly, Zhou et al. indicated that while Piezo2 is dispensable for bone development, it shares redundant functions with Piezo1 in vivo [33]. Deficiency of Piezo1 and Piezo2 in osteoblasts results in more severe bone loss in mice than deficiency of Piezo1 [33]. In vitro, Piezo1 and Piezo2 convert mechanical signals (fluid shear stress and extracellular matrix stiffness) into intracellular Ca2+ signaling that activates calcineurin, which promotes concerted activation of NFATc1 (nuclear factor of activated T-cells, cytoplasmic 1), YAP1, and β-catenin transcription factors as well as NFAT/YAP1/β-catenin complex formation [33]. This process ultimately promotes osteoblast differentiation [33].
4.4. Piezo1 and Osteoclasts
Multinucleated osteoclasts are differentiated from cells of the myeloid lineage at various stages of maturity [62]. Osteoclasts are mainly responsible for initiating normal bone remodeling and mediating bone loss in pathologic conditions by increasing their resorptive activity [63]. Attaching to the old bone area and sensing surrounding mechanical environments, osteoclasts secrete acid and protease to digest the bone matrix and form a bone absorption cavity [64][65]. During bone remodeling, osteoclasts are regulated by osteoblasts and osteocytes to maintain bone homeostasis [55][66]. Sun et al. detected the expression of Piezo1 and cationic current induced by mechanical poking on the cell membrane in the pre-osteoclast cell line RAW264.7 [49]. To test whether Piezo1 affected the bone resorption of osteoclasts, Wang et al. deleted Piezo1 from mouse osteoclasts in vivo [47]. Consequently, bone resorption and bone mass in mice with Piezo1-deficiency in osteoclasts were basically unchanged compared with control mice. These findings suggest that Piezo1 has no role in osteoclasts, but whether Piezo2 has any role in osteoclasts is unknown.
4.5. Piezo1 and Chondrocytes
| Cell Type | Piezo1’s Functions | Mechanism | Reference |
|---|---|---|---|
| Osteocytes | Promotes bone formation regulated by osteocytes | Activates downstream Wnt1 signaling and Akt signaling | [34][50] |
| Mesenchymal stem cells | Promotes the differentiation of MSC into osteoblasts | Upregulates BMP2 | [48] |
| Osteoblasts | Promotes bone formation by osteoblast; reduces cell proliferation but increases its migration ability; indirectly inhibits bone resorption of osteoclasts | Activates Piezo1-YAP1-collagen pathway; activates NFAT/YAP1/β-catenin transcription factor complex with Piezo2; activates AKT/GSK-3β/β-catenin pathway | [33][47][49][75][76][77] |
| Osteoclasts | No | No | [47] |
| Chondrocytes | Promotes chondrocyte apoptosis; promotes endochondral ossification in which chondrocytes are involved | Activates Ca2+ transient; activates MAPK/ERK1/2 pathway; activates caspase-12-dependent pathway; | [51][12][52][74] |
5. Piezo Channels and Bone Disease
Because Piezo channels play important roles in regulating bone physiology, recent studies demonstrate the involvement of Piezo channels in bone disease. Here, we mainly introduce the role of Piezo channels in osteoporosis (OP) and osteoarthritis (OA).
5.1. Osteoporosis (OP)
5.2. Osteoarthritis (OA)
| Cell Type | Piezo1’s Functions | Mechanism | Reference |
|---|---|---|---|
| Osteoporosis | Downregulation in osteocyte and/or osteoblast; promotion of bone resorption leading to imbalance among bone formation and bone resorption under mechanical unloading | Unclear | [47][34][49] |
| Osteoarthritis | Upregulation in damaged chondrocytes; promotion of apoptosis of chondrocytes; rarefication of the F-actin cytoskeleton and amplification of mechanically induced deformation microtrauma | Activates caspase-12 signaling; promotes excessive Ca2+ influx, which in turn rarefies the F-actin cytoskeleton | [51][12][93] |
References
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo proteins: Regulators of mechanosensation and other cellular processes. J. Biol. Chem. 2014, 289, 31673–31681.
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60.
- Wu, J.; Lewis, A.H.; Grandl, J. Touch, Tension, and Transduction—The Function and Regulation of Piezo Ion Channels. Trends Biochem. Sci. 2017, 42, 57–71.
- Ge, J.; Li, W.; Zhao, Q.; Li, N.; Chen, M.; Zhi, P.; Li, R.; Gao, N.; Xiao, B.; Yang, M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 2015, 527, 64–69.
- Wang, L.; Zhou, H.; Zhang, M.; Liu, W.; Deng, T.; Zhao, Q.; Li, Y.; Lei, J.; Li, X.; Xiao, B. Structure and mechanogating of the mammalian tactile channel PIEZO2. Nature 2019, 573, 225–229.
- Zhao, Q.; Zhou, H.; Chi, S.; Wang, Y.; Wang, J.; Geng, J.; Wu, K.; Liu, W.; Zhang, T.; Dong, M.Q.; et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 2018, 554, 487–492.
- Roh, J.; Hwang, S.M.; Lee, S.H.; Lee, K.; Kim, Y.H.; Park, C.K. Functional Expression of Piezo1 in Dorsal Root Ganglion (DRG) Neurons. Int. J. Mol. Sci. 2020, 21, 3834.
- Mikhailov, N.; Leskinen, J.; Fagerlund, I.; Poguzhelskaya, E.; Giniatullina, R.; Gafurov, O.; Malm, T.; Karjalainen, T.; Grohn, O.; Giniatullin, R. Mechanosensitive meningeal nociception via Piezo channels: Implications for pulsatile pain in migraine? Neuropharmacology 2019, 149, 113–123.
- LaPaglia, D.M.; Sapio, M.R.; Burbelo, P.D.; Thierry-Mieg, J.; Thierry-Mieg, D.; Raithel, S.J.; Ramsden, C.E.; Iadarola, M.J.; Mannes, A.J. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia 2018, 38, 912–932.
- Martins, J.R.; Penton, D.; Peyronnet, R.; Arhatte, M.; Moro, C.; Picard, N.; Kurt, B.; Patel, A.; Honore, E.; Demolombe, S. Piezo1-dependent regulation of urinary osmolarity. Pflugers Arch. 2016, 468, 1197–1206.
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467.
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122.
- Nonomura, K.; Woo, S.H.; Chang, R.B.; Gillich, A.; Qiu, Z.; Francisco, A.G.; Ranade, S.S.; Liberles, S.D.; Patapoutian, A. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 2017, 541, 176–181.
- Ranade, S.S.; Woo, S.H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Begay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014, 516, 121–125.
- Woo, S.H.; Ranade, S.; Weyer, A.D.; Dubin, A.E.; Baba, Y.; Qiu, Z.; Petrus, M.; Miyamoto, T.; Reddy, K.; Lumpkin, E.A.; et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014, 509, 622–626.
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. Elife 2015, 4, e07369.
- Wang, Y.; Chi, S.; Guo, H.; Li, G.; Wang, L.; Zhao, Q.; Rao, Y.; Zu, L.; He, W.; Xiao, B. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 2018, 9, 1300.
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 2011, 50, 6295–6300.
- Copp, S.W.; Kim, J.S.; Ruiz-Velasco, V.; Kaufman, M.P. The mechano-gated channel inhibitor GsMTx4 reduces the exercise pressor reflex in decerebrate rats. J. Physiol. 2016, 594, 641–655.
- Drew, L.J.; Wood, J.N. FM1-43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol. Pain 2007, 3, 1.
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488.
- Zhao, Q.; Zhou, H.; Li, X.; Xiao, B. The mechanosensitive Piezo1 channel: A three-bladed propeller-like structure and a lever-like mechanogating mechanism. FEBS J. 2019, 286, 2461–2470.
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13.
- Zhao, Q.; Wu, K.; Geng, J.; Chi, S.; Wang, Y.; Zhi, P.; Zhang, M.; Xiao, B. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron 2016, 89, 1248–1263.
- Geng, J.; Liu, W.; Zhou, H.; Zhang, T.; Wang, L.; Zhang, M.; Li, Y.; Shen, B.; Li, X.; Xiao, B. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron 2020, 106, 438–451.
- Zheng, W.; Gracheva, E.O.; Bagriantsev, S.N. A hydrophobic gate in the inner pore helix is the major determinant of inactivation in mechanosensitive Piezo channels. Elife 2019, 8, e44003.
- Burke, S.D.; Jordan, J.; Harrison, D.G.; Karumanchi, S.A. Solving Baroreceptor Mystery: Role of PIEZO Ion Channels. J. Am. Soc. Nephrol. 2019, 30, 911–913.
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746.
- Geng, J.; Zhao, Q.; Zhang, T.; Xiao, B. In Touch With the Mechanosensitive Piezo Channels: Structure, Ion Permeation, and Mechanotransduction. Curr. Top. Membr. 2017, 79, 159–195.
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282.
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121.
- Liu, L.; Zhang, Q.; Xiao, S.; Sun, Z.; Ding, S.; Chen, Y.; Wang, L.; Yin, X.; Liao, F.; Jiang, L.H.; et al. Inhibition of Shear-Induced Platelet Aggregation by Xueshuantong via Targeting Piezo1 Channel-Mediated Ca(2+) Signaling Pathway. Front. Pharmacol. 2021, 12, 606245.
- Zhou, T.; Gao, B.; Fan, Y.; Liu, Y.; Feng, S.; Cong, Q.; Zhang, X.; Zhou, Y.; Yadav, P.S.; Lin, J.; et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ss-catenin. Elife 2020, 9, e52779.
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631.
- Suchyna, T.M. Piezo channels and GsMTx4: Two milestones in our understanding of excitatory mechanosensitive channels and their role in pathology. Prog. Biophys. Mol. Biol. 2017, 130, 244–253.
- Eijkelkamp, N.; Linley, J.E.; Torres, J.M.; Bee, L.; Dickenson, A.H.; Gringhuis, M.; Minett, M.S.; Hong, G.S.; Lee, E.; Oh, U.; et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 2013, 4, 1682.
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143.
- Ren, L.; Yang, P.; Wang, Z.; Zhang, J.; Ding, C.; Shang, P. Biomechanical and biophysical environment of bone from the macroscopic to the pericellular and molecular level. J. Mech. Behav. Biomed. Mater. 2015, 50, 104–122.
- Yu, K.; Sellman, D.P.; Bahraini, A.; Hagan, M.L.; Elsherbini, A.; Vanpelt, K.T.; Marshall, P.L.; Hamrick, M.W.; McNeil, A.; McNeil, P.L.; et al. Mechanical loading disrupts osteocyte plasma membranes which initiates mechanosensation events in bone. J. Orthop. Res. 2018, 36, 653–662.
- Van Oers, R.F.; Wang, H.; Bacabac, R.G. Osteocyte shape and mechanical loading. Curr. Osteoporos. Rep. 2015, 13, 61–66.
- Plotkin, L.I.; Bruzzaniti, A. Molecular signaling in bone cells: Regulation of cell differentiation and survival. Adv. Protein Chem. Struct. Biol. 2019, 116, 237–281.
- Heino, T.J.; Hentunen, T.A. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2008, 3, 131–145.
- Hinton, P.V.; Rackard, S.M.; Kennedy, O.D. In Vivo Osteocyte Mechanotransduction: Recent Developments and Future Directions. Curr. Osteoporos. Rep. 2018, 16, 746–753.
- Yan, Y.; Wang, L.; Ge, L.; Pathak, J.L. Osteocyte-Mediated Translation of Mechanical Stimuli to Cellular Signaling and Its Role in Bone and Non-bone-Related Clinical Complications. Curr. Osteoporos. Rep. 2020, 18, 67–80.
- Guilak, F. Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2011, 25, 815–823.
- Gao, Y.; Liu, S.; Huang, J.; Guo, W.; Chen, J.; Zhang, L.; Zhao, B.; Peng, J.; Wang, A.; Wang, Y.; et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed. Res. Int. 2014, 2014, 648459.
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282.
- Sugimoto, A.; Miyazaki, A.; Kawarabayashi, K.; Shono, M.; Akazawa, Y.; Hasegawa, T.; Ueda-Yamaguchi, K.; Kitamura, T.; Yoshizaki, K.; Fukumoto, S.; et al. Piezo type mechanosensitive ion channel component 1 functions as a regulator of the cell fate determination of mesenchymal stem cells. Sci. Rep. 2017, 7, 17696.
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife 2019, 8, e47454.
- Sasaki, F.; Hayashi, M.; Mouri, Y.; Nakamura, S.; Adachi, T.; Nakashima, T. Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem. Biophys. Res. Commun. 2020, 521, 806–813.
- Li, X.F.; Zhang, Z.; Chen, Z.K.; Cui, Z.W.; Zhang, H.N. Piezo1 protein induces the apoptosis of human osteoarthritis-derived chondrocytes by activating caspase-12, the signaling marker of ER stress. Int. J. Mol. Med. 2017, 40, 845–853.
- Li, X.F.; Zhang, Z.; Li, X.D.; Wang, T.B.; Zhang, H.N. Mechanism of the Piezo1 protein-induced apoptosis of the chondrocytes through the MAPK/ERK1/2 signal pathway. Zhonghua Yi Xue Za Zhi 2016, 96, 2472–2477.
- Moriishi, T.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Maeno, T.; Kawai, Y.; Komori, H.; Komori, T. Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS ONE 2012, 7, e40143.
- Li, X.; Kordsmeier, J.; Xiong, J. New Advances in Osteocyte Mechanotransduction. Curr. Osteoporos. Rep. 2021, 19, 101–106.
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238.
- Zhang, Z.H.; Jia, X.Y.; Fang, J.Y.; Chai, H.; Huang, Q.; She, C.; Jia, P.; Geng, C.; Xu, W. Reduction of SOST gene promotes bone formation through the Wnt/beta-catenin signalling pathway and compensates particle-induced osteolysis. J. Cell Mol. Med. 2020, 24, 4233–4244.
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784.
- Oreffo, R.O.; Cooper, C.; Mason, C.; Clements, M. Mesenchymal stem cells: Lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005, 1, 169–178.
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139.
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360.
- Kokabu, S.; Lowery, J.W.; Jimi, E. Cell Fate and Differentiation of Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3753581.
- De Vries, T.J.; Schoenmaker, T.; Hooibrink, B.; Leenen, P.J.; Everts, V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J. Leukoc. Biol. 2009, 85, 919–927.
- Back, S.H.; Adapala, N.S.; Barbe, M.F.; Carpino, N.C.; Tsygankov, A.Y.; Sanjay, A. TULA-2, a novel histidine phosphatase, regulates bone remodeling by modulating osteoclast function. Cell Mol. Life Sci. 2013, 70, 1269–1284.
- Boyce, B.F.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180.
- Kurata, K.; Uemura, T.; Nemoto, A.; Tateishi, T.; Murakami, T.; Higaki, H.; Miura, H.; Iwamoto, Y. Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J. Bone Miner. Res. 2001, 16, 722–730.
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. Bonekey Rep. 2014, 3, 481.
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. 1996, 81, 535–545.
- Zhou, Q.; Zhang, J.H.; Yuan, S.; Shao, J.H.; Cai, Z.Y.; Chen, S.; Cao, J.; Wu, H.S.; Qian, Q.R. A New Insight of Kartogenin Induced the Mesenchymal Stem Cells (MSCs) Selectively Differentiate into Chondrocytes by Activating the Bone Morphogenetic Protein 7 (BMP-7)/Smad5 Pathway. Med. Sci. Monit. 2019, 25, 4960–4967.
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224.
- Servin-Vences, M.R.; Moroni, M.; Lewin, G.R.; Poole, K. Direct measurement of TRPV4 and PIEZO1 activity reveals multiple mechanotransduction pathways in chondrocytes. Elife 2017, 6, e21074.
- Lawrence, K.M.; Jones, R.C.; Jackson, T.R.; Baylie, R.L.; Abbott, B.; Bruhn-Olszewska, B.; Board, T.N.; Locke, I.C.; Richardson, S.M.; Townsend, P.A. Chondroprotection by urocortin involves blockade of the mechanosensitive ion channel Piezo1. Sci. Rep. 2017, 7, 5147.
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321.
- Du, G.; Li, L.; Zhang, X.; Liu, J.; Hao, J.; Zhu, J.; Wu, H.; Chen, W.; Zhang, Q. Roles of TRPV4 and piezo channels in stretch-evoked Ca(2+) response in chondrocytes. Exp. Biol. Med. 2020, 245, 180–189.
- Hendrickx, G.; Fischer, V.; Liedert, A.; von Kroge, S.; Haffner-Luntzer, M.; Brylka, L.; Pawlus, E.; Schweizer, M.; Yorgan, T.; Baranowsky, A.; et al. Piezo1 Inactivation in Chondrocytes Impairs Trabecular Bone Formation. J. Bone Miner. Res. 2021, 36, 369–384.
- Yan, L.; Jiang, J.; Ma, C.; Li, R.; Xia, Y. Effect of knocking down Piezo1 mechanically sensitive protein on migration of MC3T3-E1 osteoblast cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 28–34.
- Yoneda, M.; Suzuki, H.; Hatano, N.; Nakano, S.; Muraki, Y.; Miyazawa, K.; Goto, S.; Muraki, K. PIEZO1 and TRPV4, which Are Distinct Mechano-Sensors in the Osteoblastic MC3T3-E1 Cells, Modify Cell-Proliferation. Int. J. Mol. Sci. 2019, 20, 4960.
- Song, J.; Liu, L.; Lv, L.; Hu, S.; Tariq, A.; Wang, W.; Dang, X. Fluid shear stress induces Runx-2 expression via upregulation of PIEZO1 in MC3T3-E1 cells. Cell Biol. Int. 2020, 44, 1491–1502.
- Montgomery, G.; Abt, G.; Dobson, C.; Smith, T.; Evans, W.; Ditroilo, M. The mechanical loading and muscle activation of four common exercises used in osteoporosis prevention for early postmenopausal women. J. Electromyogr. Kinesiol. 2019, 44, 124–131.
- Li, X.; Liu, D.; Li, J.; Yang, S.; Xu, J.; Yokota, H.; Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019, 33, 8913–8924.
- Braith, R.W.; Conner, J.A.; Fulton, M.N.; Lisor, C.F.; Casey, D.P.; Howe, K.S.; Baz, M.A. Comparison of alendronate vs alendronate plus mechanical loading as prophylaxis for osteoporosis in lung transplant recipients: A pilot study. J. Heart Lung Transplant. 2007, 26, 132–137.
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2021, 1–13.
- Xiao, Z.; Zhang, S.; Mahlios, J.; Zhou, G.; Magenheimer, B.S.; Guo, D.; Dallas, S.L.; Maser, R.; Calvet, J.P.; Bonewald, L.; et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J. Biol. Chem. 2006, 281, 30884–30895.
- Xiao, Z.; Dallas, M.; Qiu, N.; Nicolella, D.; Cao, L.; Johnson, M.; Bonewald, L.; Quarles, L.D. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 2011, 25, 2418–2432.
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730.
- Dalagiorgou, G.; Piperi, C.; Georgopoulou, U.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol. Life Sci. 2013, 70, 167–180.
- Qiu, N.; Xiao, Z.; Cao, L.; David, V.; Quarles, L.D. Conditional mesenchymal disruption of pkd1 results in osteopenia and polycystic kidney disease. PLoS ONE 2012, 7, e46038.
- Xiao, Z.; Zhang, S.; Cao, L.; Qiu, N.; David, V.; Quarles, L.D. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J. Biol. Chem. 2010, 285, 1177–1187.
- Xiao, Z.; Cao, L.; Liang, Y.; Huang, J.; Stern, A.R.; Dallas, M.; Johnson, M.; Quarles, L.D. Osteoblast-specific deletion of Pkd2 leads to low-turnover osteopenia and reduced bone marrow adiposity. PLoS ONE 2014, 9, e114198.
- Xiao, Z.; Quarles, L.D. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev. Endocr. Metab. Disord. 2015, 16, 115–129.
- Mesner, L.D.; Ray, B.; Hsu, Y.H.; Manichaikul, A.; Lum, E.; Bryda, E.C.; Rich, S.S.; Rosen, C.J.; Criqui, M.H.; Allison, M.; et al. Bicc1 is a genetic determinant of osteoblastogenesis and bone mineral density. J. Clin. Invest. 2014, 124, 2736–2749.
- Leuenroth, S.J.; Okuhara, D.; Shotwell, J.D.; Markowitz, G.S.; Yu, Z.; Somlo, S.; Crews, C.M. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc. Natl. Acad. Sci. USA 2007, 104, 4389–4394.
- He, B.H.; Christin, M.; Mouchbahani-Constance, S.; Davidova, A.; Sharif-Naeini, R. Mechanosensitive ion channels in articular nociceptors drive mechanical allodynia in osteoarthritis. Osteoarthr. Cartil. 2017, 25, 2091–2099.
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2001611118.
- Xu, B.; Xing, R.; Huang, Z.; Yin, S.; Li, X.; Zhang, L.; Ding, L.; Wang, P. Excessive mechanical stress induces chondrocyte apoptosis through TRPV4 in an anterior cruciate ligament-transected rat osteoarthritis model. Life Sci. 2019, 228, 158–166.




