
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatiana Montoya Garcia | + 7151 word(s) | 7151 | 2021-05-27 07:44:12 | | | |
| 2 | Vivi Li | Meta information modification | 7151 | 2021-06-28 04:00:45 | | |
Video Upload Options
Iridoids, which have beneficial health properties, include a wide group of cyclopentane [c] pyran monoterpenoids present in plants and insects. The cleavage of the cyclopentane ring leads to secoiridoids. Mainly, secoiridoids have shown a variety of pharmacological effects including anti-diabetic, antioxidant, anti-inflammatory, immunosuppressive, neuroprotective, anti-cancer, and anti-obesity, which increase the interest of studying these types of bioactive compounds in depth. Secoiridoids are thoroughly distributed in several families of plants such as Oleaceae, Valerianaceae, Gentianaceae and Pedialaceae, among others. Specifically, Olea europaea L. (Oleaceae) is rich in oleuropein (OL), dimethyl-OL, and ligstroside secoiridoids, and their hydrolysis derivatives are mostly OL-aglycone, oleocanthal (OLE), oleacein (OLA), elenolate, oleoside-11-methyl ester, elenoic acid, hydroxytyrosol (HTy), and tyrosol (Ty). These compounds have proved their efficacy in the management of diabetes, cardiovascular and neurodegenerative disorders, cancer, and viral and microbial infections. Particularly, the antioxidant, anti-inflammatory, and immunomodulatory properties of secoiridoids from the olive tree (Olea europaea L. (Oleaceae)) have been suggested as a potential application in a large number of inflammatory and reactive oxygen species (ROS)-mediated diseases.
1. Introduction
1.1. Structure and Classification
-
Group 1: C8 iridoids (di-nor-iridoids)
-
Group 2: C9 iridoids (nor-iridoids)
-
Group 3: C10 iridoids, which occur mainly as glycosides
-
Group 4: Aglycones and some iridoids included in the other three groups lacking a sugar residue in their structure
-
Group 5: Iridoids derivatives. This group comprises compounds derived from the opening of the pyran ring
-
Group 6: Included bis-iridoids as a result of condensation of two monomers, (a) directly as in iridolinalin A, or (b) through a sugar residue as in globuloside A.
-
Simple secoiridoids: Generally, for the simple secoiridoids, positions C7 and C11 have either a free carboxylic acid group or a methyl ethyl ester derivative of the acid. The configurations of the positions C1 and C5 are S.
-
Conjugated secoiridoids: This group of compounds is the most numerous secoiridoids isolated from the Oleaceae family. The name of the class derives from the type of compound that is linked or conjugated to the secoiridoid nucleus. Based on this, this class is further categorized into seven subgroups: aromatic-conjugated, sugar-conjugated, terpene-conjugated, cyclopentane-conjugated, coumarin-conjugated, lignans-conjugated, and other secoiridoids. Normally, the conjugations occur in C7 due to this position, which is is usually oxidized to a carboxylic acid and esterified with diverse groups.
-
10-Oxyderivative of oleoside secoiridoids: This group contains the oleoside nucleus with distinct structural differences. The C8 and C9 positions exist as double bonds, with a hydroxy group at the C8 position or an ester formed by an oxygen atom with different groups. A total of 40 10-Oxyderivative of oleoside secoiridoids have been isolated from the Oleaceae family.
-
Z-Secoiridoids: This class of secoiridoids is characterized by the presence of double-bond geometry at the C8 in Z-configuration; however, only five compounds have been isolated from the Oleaceae family.
-
Secologanosides and oxidized secologanoside secoiridoids: Compounds of this class are based on the secologanoside nucleus. They are differentiated by the positions on the C–C double bond between C8 and C10 and C10 oxidation level.
1.2. Main Naturally Occurring Iridoids and Secoiridoids Present in Olea europaea L
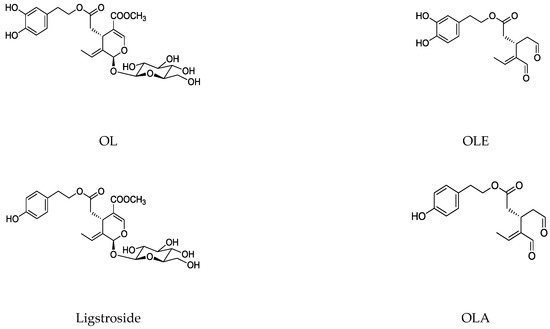
1.3. Biosynthesis and Biotransformation of Secoiridoids in Olive Tree
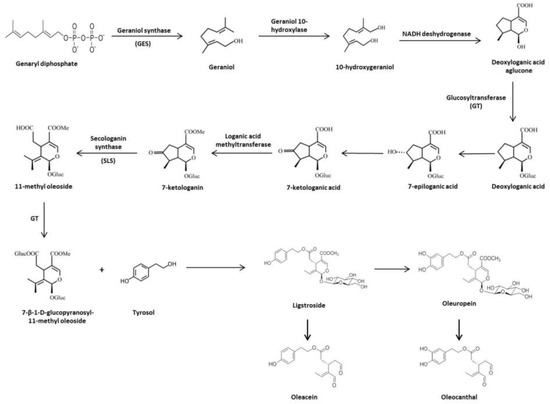
2. Protective Role of the Olive Tree Secoiridois in Diseases with an Important Pathogenic Contribution of Oxidative and Peroxidative Damage
2.1. Olive Tree Secoiridoids and Cancer
| Phenolic Compound | Cell Line | Concentration | Effects | Reference |
|---|---|---|---|---|
 OL OL |
NL-Fib: normal human skin fibroblasts; LN-18: poorly differentiated glioblastoma; TF-1a: erythroleukemia; 786O: renal cell adenocarcinoma; T-47D: infiltrating ductal carcinoma of breast-pleural effusion; MCF-7: human breast cancer; RPMI-7951: malignant melanoma skin-lymphoide metastasis; LoVo: colorectal adenocarcinoma-supraclavicular region metastasis | 0.005%, 0.01% and 0.025% of OL in fibroblast tissue culture medium | OL inhibited cell growth, motility and invasiveness | [35] |
| HT29 and SW260 human colon adenocarcinoma cell line | [0–100 µM] | OL might induce anti-proliferative and pro-apoptotic effects | [36] | |
| HT29 | 200, 400, and 800 µM | OL limited the growth and induced apoptosis via the p53 pathway | [37] | |
| MDA-MB-231 human breast cancer cell line | 200 µg/mL | OL produced the up-regulating of TIMPs gene expression and the down-regulation MMPs overexpression gene | [38] | |
| MDA-MB-231; MCF-10A and MCF-7 human breast cancer cell lines | [0–300 µM] | OL exhibited specific cytoxicity against breast cancer cells, which is probably mediated through the induction of apoptosis via mitochondrial pathway | [39] | |
| SKBR3 breast cancer cell line | 100 µM | OL worked as G-protein-coupled receptor (GPER) inverse agonists in estrogen receptor (ER)-negative and GPER-positive SKBR3 | [40] | |
| MCF-7 | 100 and 200 µM | OL induced apoptosis in breast tumor cells via p53-dependent pathway | [41] | |
| MDA-MB-231 and MCF-7 | [0–100 µM] | OL inhibited the viability of breast cancer cells and induced apoptosis via modulating NF-κB activation cascade | [42] | |
| MCF-7 | [0–1200 µg/mL] | OL suppressed cells migration through suppression of epithelial-mesenchymal transition and could reduce DOX-induced side effects by reducing its effective dose | [43] | |
| MCF-7 | [0–100 µM] | OL decreased the expression of both HDAC2 and HDAC3, induced apoptosis, and retarded cell migration and cell invasion in a dose-dependent manner | [44] | |
| MCF-7 | 200, 400, 600, and 1000 µM | OL inhibited the proliferation and invasion of cells by inducing apoptosis | [45] | |
| MDA-MB-231 | [0–100 µM] | OL reduced cell viability in a dose-dependent manner; suppressed HGF or 3-MA, and induced cell migration and invasion | [46] | |
| MCF-7 | [0–250 µM] | OL inhibited protein tyrosine phosphatase 1B (PTB1B) | [47] | |
| HepG2 and Huh7 human HCC cell lines | [0–100 µM] | OL induced apoptosis in HCC cells via the suppression of PI3K/Akt | [48] | |
| HepG2 | 100, 200 and 300 µM | OL could control the influencing of pro-nerve growth factor (NGF) and NGF balance via affecting MMP-7 activity without affecting the gene expression of NGF in HCC. | [49] | |
| LNCaP human prostate cancer androgen-responsive and DU145 androgen non-responsive cell lines | 100 and 500 µM | OL reduced cell viability and induced thiol group modification | [50] | |
| TCP-1 and BCPAP thyroid tumor cell line | 10, 50, and 100 µM | OL was able to inhibit in vitro thyroid cancer cell proliferation acting on the growth-promoting signal pathway | [51] | |
| HeLa human cervical carcinoma cell line | 150 and 200 µM | OL-induced apoptosis was activated by the JNK/SPAK signal pathway | [52] | |
| SH-SY5Y human neuroblastoma cell line | 350 µM | OL caused cell cycle arrest by down-regulating CyclinD1, CyclinD2, CyclingD3, CDK4, and CDK6 and up-regulating p53 and CDKN2A, CDKN2B, CDKN1A gene expressions. OL also induced apoptosis | [53] | |
| U251 and A172 human glioma cancer cell lines | 0, 200, and 400 µM | OL inhibited cell viability and reduced the expression levels of MMP-2 and MMP-9. In addition, a specific PI3K inhibitor enhanced the pro-apoptotic and anti-invasive effects induced by OL | [54] | |
| HNE1 and HONE1 human nasopharyngeal carcinoma (NPC) cell lines | 0 and 200 µM | OL treatments reduced the activity of the HIF-1α-miR-519d-PDRG1 pathway, which is essential to the radio-sensitizing effect of OL | [55] | |
| A549 human non-small cell lung cancer (NSCLC) | [0–200 µM] | OL caused a decrease in mithocondrial membrane potential, increase in Bax/Bcl2 ratio, release of mithocondrial cytochrome C, and activation of caspase 9 and caspase 3 | [56] | |
| H1299 lung cancer cell line | [0–200 µM] | OL-induced apoptosis via the mitochondrial apoptotic cascade was activated by the p38 MAPK signaling pathway in H1299 cells | [57] | |
| A549 and BEAS-2B human noncancerous cell line | 50 and 150 µM | OL induced apoptosis in A549 cells | [58] | |
| MIA PaCa-2, BxPC-3, and CFPAC-1 pancreatic cancer and HPDE non-tumorigenic pancreas cell lines | 200 µM | OL arrested cell cycle, increased the Bax/Bcl-2 ratio, increased the activation of caspase 3/7, and induced apoptosis in MIA-PaCa-2 | [59] | |
| A375 human melanoma cell line | [250–500 µM] | OL was able to stimulate apoptosis (500 µM), while at a dose of 250 µM it affected cell proliferation and induced the down-regulation of the pAkt/pS6 pathway | [60] | |
| OE-19 human esophagical cancer (EC) cell line | 200 µM | OL inhibited the growth of EC cells as well as inhibiting HIF-1α and up-regulating BTG anti-proliferation F factor 3 (BTG3) expressions | [61] | |
| 143B human osteosarcoma (OS) cell line | 100 µM | OL showed alone and in combination with 2-methoxyestradiol a potent anti-cancer potential in highly metastatic OS cell | [62] | |
| AGS Human gastric adenocarcinoma cell line | [0–1000 µg/mL] | Magnetic nano-OL could trigger apoptosis in the AGS cell line | [63] | |
 OL-aglycone OL-aglycone |
SH-SY5Y and RIN-5F insulinoma cell lines | 100 µM | OL-aglycone triggered autophagy in cultured cells through the Ca2+-CAMKKβ–AMPK axis. | [64] |
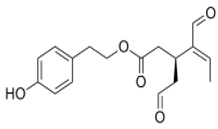 OLE OLE |
HT29 and HCT-116 human colon adenocarcinoma cell line | 1, 2, 5, and 10 µg/mL | OLE produced an inhibition of AP1 activity and cyclooxygenase 2 (COX2) expression in HT29 cells | [65] |
| MDA-MB-231, MCF-7, and PC3 prostate cancer cell lines | [0–20 µM] | OLE inhibited the proliferation, migration, and invasion of the epithelial human breast and prostate cancer cell lines and demonstrated anti-angiogenic activity | [66] | |
| BT-474, MDA-MB-231, and MCF-7 | [0–60 µM] | OLE reduced the c-Met kinase activity, cell growth, migration, and invasion of breast cancer cells and induced G1 cell cycle arrest and apoptosis, as well as, inhibited c-Met-dependent signaling | [67] | |
| MDA-MB-231 | [0–10 µM] | OLE showed strong anti-proliferative and down-regulated the expression of phosphorylated mTOR | [68] | |
| BT-474 | [0–100 µg/mL] | OLE reduced breast cancer progression and locoregional recurrence models | [69] | |
| MDA-MB-231 | 5 mg/mL | OLE was able to control breast cancer progression | [70] | |
| BT-474 and MDA-MB-231 | [0–200 µM] | OLE with the dual HER2/EGFR inhibitor, LP, induced synergistic tumor growth inhibition | [71] | |
| MCF-10A, MDA-MB-231, and MCF-7 | 1, 10, and 20 µM | OLE could be responsible for the selective activation of TRCP6-dependent Ca2+ influx and TRCP6 down-regulation at low µM concentrations | [72] | |
| Huh-7, HepG2, and HCCLM3 HCC cancer cell lines | [0–80 µM] | OLE inhibited proliferation and cell cycle progression and also inhibited HCC cell migration and invasion | [73] | |
| Huh-7, HepG2, and HCCLM3 | 5 and 10 µM | OLE reduced cell proliferation and increased cell death | [74] | |
| U937 hystocytic lymphoma cancer cell line | 30 µM | OLE significantly inhibited the expression of Hsp90, a chaperone with a key role in cancer and neurodegeneration | [75] | |
| A375: A2058; HUVEC and HaCat cancer cell lines | [0–60 µM] | OLE suppressed STAT3 phosphorylation, decreased STAT3 nuclear localization, and inhibited STAT3 transcriptional activity | [76] | |
| Inmortalized human keratinocytes stimulated with epidermal growth factor | [0–100 µM] | OLE promoted the inhibition of ERK and Akt phosphorylation and the suppression of B-raf expression | [77] | |
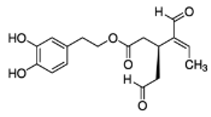 OLA OLA |
Inmortalized human keratinocytes stimulated with epidermal growth factor | [0–100 µM] | OLA promoted the inhibition of Erk and Akt phosphorylation and the suppression of B-raf expression | [77] |
| HL60 human promyelocytic leukemia cell line | [0–10 µM] | OLA reduced the DNA damage at concentrations as low as 1 µM when co-incubated in the medium with H2O2 | [78] | |
| NCI-H929; RPMI-8226; U266; MM1S and IIN3 human MM cancer cell lines | 2.5, 5 and 10 µM | OLA elicited significant antitumor activity by promoting cell cycle arrest and apoptosis either with a simple agent or in combination with Carfilzomib | [79] |
| Phenolic Compound | Animal Model | Doses | Effects | Reference |
|---|---|---|---|---|
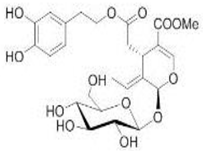 OL OL |
Swiss albino with soft tissue sarcomas | 1% OLE in drinking water | OL inhibited cell growth, motility, and invasiveness | [35] |
| Male hairless mice (5 weeks old) were UVB irratied (36–180 mJ/cm2) | 10 and 25 mg/Kg/day | OL increased the skin thickness and reductions in skin elasticity, skin carcinogenesis, and tumor growth | [80] | |
| DSS-induced CRC in C57BL/6 mice | 50 and 100 mg/Kg | OL prevented the development of colonic neoplasia in by ameliorating colon inflammatory processes and limiting the activation of the main transcription factors involved | [81] | |
| Male Sprague–Dawley rats that received an injection of cisplatin (7 mg/Kg) | 50, 100, and 200 mg/Kg/day | OL enhanced antioxidant activity and prevented oxidative stress, which it turn reduced 8-hydroxy-2’deoxy-guanosine (8-OH-dG) levels in lymphocytes of cisplatin-treated animals | [82] | |
| HNE1 and HONE1 injected into 6–8-week-old BalB/c mice | [0–200 µM] | OL was a radiation-sensitizing agent of NPC cells in an in vivo model | [55] | |
| Four-week-old C57BL/6N mice with HFD with or without OL and which were injected with B16F10 melanoma cells | 0.02% and 0.04% enriched-diets | OL suppressed HFD-induced solid tumor growth and reduced HFD-induced expression of angiogenesis, lymphangiogenesis, and hypoxia markers | [83] | |
| Male Sprague–Dawley rats that received an injection of cisplatin (7 mg/Kg) | 50, 100, and 200 mg/Kg/day | OL significantly decreased the formations of DNA damage and the level of malondialdehyde (MDA), and it increased the levels of total antioxidant status in pancreas tissue samples | [84] | |
| BalB/c OlaHsd-foxn1 injected with MDA-MB-231 | 50 mg/Kg | The combined treatment with OL and DOX downregulated the antiapoptosis and proliferation protein, nuclear transcription factor-kappa B (NF-κB), and its main oncogenic target Cyclin D1. It also inhibited the expression of Bcl-2 | [85] | |
| Severe combined immunodeficiency mice (6 weeks-old) that received a subcutaneous injection of OE-19 cancer cells | 200 µM | OL inhibited the growth of xenograft EC tumor as well as inhibited HIF-1α and upregulated B-cell translocation gene 3 (BTG3) expressions | [61] | |
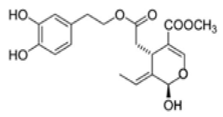 OL-aglycone OL-aglycone |
Transgenic hemizygous CRND8 mice harboring a double-mutant gene of APP695 and wild-type control lettermates with 4 and 10 months of age | 100 µM | In OL-fed animals, there was a reduction of phospho-mTOR immunoreactivity and phosphorylated mTOR substrate p70 S6K levels | [64] |
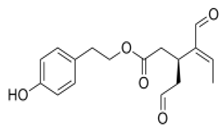 OLE OLE |
Swiss albino mice (6 weeks old) | 10 mg/Kg/day | OLE reduced breast cancer progression and locoregional recurrence models | [69] |
| Female athymic nude mice (Foxn1nu/Foxn1+) (4-5 weeks-old) inyected with BT-474 and MDa-MB-231 | 10 mg/Kg/day | OLE inhibited locoregional recurrence in luminal HER2+/ER+ BT-474 tumors | [86] | |
| Orthotopic tumor model of HCC in BalB/c mice | 0, 5 and 10 mg/Kg/day | OLE suppressed tumor growth and impeded HCC metastasis in an in vivo lung metastasis model. OLE inhibited STAT3 activation and increased the activity of protein tyrosine hosphatase | [73] |
2.2. Olive Tree Secoiridoids and Cardiovascular Diseases
| Phenolic Compound | Cell Line | Concentration | Effects | Reference |
|---|---|---|---|---|
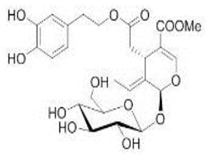 OL OL |
Healthy human LDL | 10 mM | OL inhibited LDL levels, lipid peroxides, malondial dehydelysine, 4-hydroxynonenal lysine adducts expression | [91] |
| LPS-stimulates mouse macrophages | OL reduced superoxide anion generation, neutrophils respiratory burst, and hypochlorous acid | [92] | ||
| Endothelial progenitors cells (CD31+ and VEGFR-2+) | [1–10 μM] | OL reduced senescent cells and reactive oxygen species (ROS) formation; restoration of migration, adhesion, tube formation, and the up-regulation of Nrf-2 and HO-1 expressions. | [93] | |
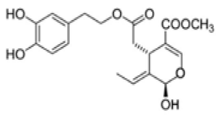 OL-aglycone OL-aglycone |
Human umbilical vascular endotelial cells | 5 and 25 mM | OL-aglycone reduced cell surface expressions and mRNA levels of ICAM-1 and VCAM-1 | [94] |
| Mouse atrial myocites HL-1 | 60 mM | OL-aglycone inhibited tranthyretin toxicity | [95] | |
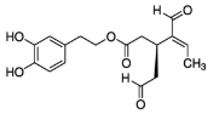 OLA OLA |
Human neutrophils and monocytes | [1–10 µM] | OLA proved to be stronger in the reduction of formyl-met-leu-phenylalanine and phorbol-myristate-acetate-induced oxidative bursts in neutrophils and myeloperoxidase release | [96] |
| Human neutrophils | 50 and 100 mM | OLA reduced elastase release, IL-8, MMP-9, and NEP activity | [97] | |
| Human macrophages | 10–20 mM | OLA increased IL-10, HO-1, and CD163 expression | [98] |
| In Vivo Studies | ||||
|---|---|---|---|---|
| Phenolic compound | Animal Model | Doses | Effects | Reference |
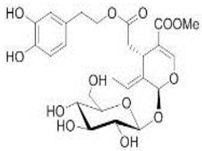 OL OL |
Ischemia–reperfusion in insolated rat hearts | 20 μg/g | OL reduced the creatine kinase, glutathione release, membrane lipid peroxidation | [99] |
| Ischemic-treated hypercholesterolemic rabbits | 10 or 20 mg/Kg/day | OL reduced the infarct size, total cholesterol, triglyceride concentration, and lipid peroxidation | [100] | |
| Doxorubicin-induced acute cardiotoxicity rats | 100 or 200 mg/Kg | OL modulated the CPK, lactate deshydrogenase, aspartate and alanine aminotransferase, and lipid peroxidation | [101] | |
| Doxorubicin-induced acute cardiotoxicity in rats | 100 or 200 mg/Kg | OL reduced the acetate and succinate levels. Restore metabolic changes | [102] | |
| Doxorubicin-induced chronic cardiomyopathy in rats | 1000 or 2000 mg/Kg | OL controlled cardiac histopathology, nitro-oxidative stress, IL-6, myocardial metabolomics | [103] | |
| Rabbit model of atherosclerosis | 100 mg/Kg | OL decreased lipids, cholesterol, LDL levels, TNF-α, NF-kB, ICAM-1, and VCAM-1 expressions | [104] | |
| Obesity-induced cardiac metabolic changes | 0.023 mg/Kg/day | OL increased oxygen consumption, fat oxidation, and myocardial β-hydroxyacyl coenzyme A dehydrogenase activity and the up-regulation of antioxidant enzyme expression | [105] | |
| Renovascular hypertension and diabetes 2 rats | 20, 40, or 60 mg/Kg/day | OL reduced blood pressure, blood glucose, serum total cholesterol, LDL, and triglycerides levels. Raised HDL levels. | [106] | |
| Diabetic hypertensive rats | 20, 40, or 60 mg/Kg/day | OL lowered blood pressure, MDA, creatine kinase, and the induction of HDL levels | [107] | |
| Diabetic hypertensive rats | 20, 40, or 60 mg/Kg/day | OL decreased blood pressure, glucose, and serum MDA levels. OL increased of HDL and erythrocyte SOD | [108] | |
| Spontaneous hypertensive rats | 10 mg/Kg | OL reduced the oxidative stress, carotid and renal hemodynamics, blood pressure, and heart rate | [109] | |
| Rats fed with high-cholesterol diet | 3 mg/Kg | OL modulated total cholesterol, triglycerides, LDL and HDL levels, and liver antioxidant enzymes | [110] | |
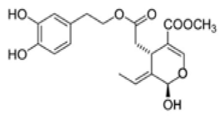 OL-aglycone OL-aglycone |
Neonatal rats ventricular myocytes with MAO-A enzyme overexpressed | 100 μM | OL-aglycone decreased oxidative stress, autophagic flux blockade and cell necrosis | [111] |
| Mature and progenitor endotelial cells | 10 μM | OL-aglycone down-regulated NF-kB, IL-8, vascular endothelial growth factor (VEGF), MMP-2, and MMP-9 | [112] | |
| Rats fed with high-cholesterol diet | 3 mg/Kg | OL-aglycone modulated total cholesterol, triglycerides, LDL and HDL levels, and liver antioxidant enzymes | [110] | |
| Clinical Trials | ||||
| Phenolic Compound | Experimental System | Concentration | Effects | Reference |
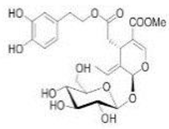 OL OL |
232 hypertensive patients | 500 mg twice daily | OL lowered systolic and diastolic blood pressure, triglycerides, and LDL levels | [113] |
2.3. Olive Tree Secoiridoids and Neurodegeneration
| Phenolic Compound | Cell Line | Concentration | Effects | Reference |
|---|---|---|---|---|
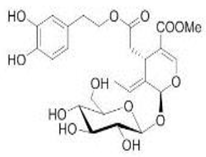 OL OL |
6-OHDA-induced toxicity in rat adrenal pheochromocytoma (PC12) cells | 20 and 25 μg/mL | OL decreased cell damage and reduce biochemical markers of PC12 cell death | [129] |
| PC12 cells exposed to the potent parkinsonian toxin 6-OHDA | 10 −12 M | OL showed neuroprotective effects in an in vitro model of PD when administered preventively as a pretreatment. OL significantly decreased neuronal death. OL could also reduce the mitochondrial production of ROS resulting from blocking SOD activity | [130] | |
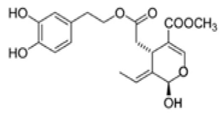 OL-aglycone OL-aglycone |
SH-SY5Y | [0–25 µM] | OL-aglycone prevented the growth of toxic Aβ1-42 oligomers and blocked their successive growth into mature fibrils following its interaction with the peptide N-terminus | [131] |
| Exposure of SH-SY5Y cells with Aβ42 | [10–1000 µM] | OL were able to attenuate cell death caused by Aβ42, copper-Aβ42, and [laevodihydroxyphenylalanine (l-DOPA)] l-DOPA-Aβ42-induced toxicity after 24 h | [132] | |
| NBM of adult male Wistar rats | 450 µM | An apparent reduction in the amount of soluble A11-positive oligomers was detected in the NBM injected with Aβ42 aggregated with OL as compared with the NBM injected with Aβ42 alone | [133] | |
 OLE OLE |
Mouse brain endothelial cells (bEnd3) | 25 and 50 µM | Treatment of bEnd3 cells with OLE resulted in significant increase in P-gp and LRP1 levels | [131] |
| Phenolic Compound. | Animal Model | Doses | Effects | Reference |
|---|---|---|---|---|
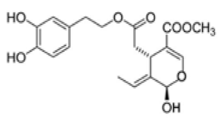 OL-aglycone OL-aglycone |
Double transgenic TgCRND8 mice, a model of amyloid-ß deposition | 8 weeks dietary supplementation of OL-aglycone (50 mg/Kg of diet) | Dietary supplementation of OL-aglycone strongly improved the cognitive performance of young/middle-aged TgCRND8 mice, with respect to age-matched littermates with unsupplemented diet | [134] |
| Transgenic mice (APPswe/PS1dE9) | 50 mg/Kg of OL-aglycone containing olive leaf extracts (OLE) from 7 to 23 weeks of age. | Treatment mice (OL-aglycone) were showed significantly reduced amyloid plaque deposition (p < 0.001) in cortex and hippocampus in comparison | [132] | |
| Transgenic CL2006 and CL4176 strains of C. elegans | 50 and 100 µM | OL-aglycone-fed CL2006 worms displayed reduced Aβ plaque deposition, less abundant toxic Aβ oligomers, remarkably decreased paralysis, and increased lifespan | [135] | |
| Systemic amyloidosis murine model | 15 µM | OL-aglycone hindered amyloid aggregation of Aβ(1-42) and its cytotoxicity and eliminated the appearance of early toxic oligomers, favoring the formation of stable harmless protofibrils, which were structurally different from the typical Aβ(1-42) fibrils | [136] | |
| TgCRND8 mice | 50 mg/Kg of diet during 8 weeks | OL-aglycone was active against glutaminylcyclase-catalyzed pE3-Aß generation, reducing enzyme expression and interfering both with Aß42 and pE3-Aß aggregation | [133] | |
| TgCRND8 (Tg) mice AD | Diet supplementation with OL-aglycone at 12.5 or 0.5 mg kg-1of diet | An OL-aglycone supplementation diet and the mix of polyphenols were found to improve significantly cognitive functions (p < 0.0001). Aß42 and pE-3Aß plaque area and number were significantly reduced in the cortex | [137] | |
 OLE OLE |
5xFAD mouse model of AD | EVOO rich with OLE | EVOO-rich OLE consumption in combination with donepezil significantly reduced Aβ load and related pathological changes | [138] |
| TgSwDI mice | Daily i.p. with 5 mg/Kg OLE at 4 age of months and continued for 4 weeks. | OLE significantly decreased amyloid load in the hippocampal parenchyma and microvessels, which was associated with enhanced cerebral clearance of Aβ across the BBB | [139] | |
| C57BL/6 wild-type male mice | 10 mg/Kg of OLE twice daily from 7 to 8 weeks of age andcontinued for 2 weeks (i.p.) | OLE enhanced clearance of Aβ from the brain. A significant increase in the expression of P-gp and LRP1 was also observed in the brain microvessels | [140] |
References
- Boros, C.A.; Stermitz, F.R. Iridoids. updated part. J. Nat. Prod. 1990, 53, 1055–1147.
- El-Naggar, L.J.; Beal, J.L. Iridoids: A Review. J. Nat. Prod. 1980, 43, 649–707.
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally Occurring Secoiridoids and Bioactivity of Naturally Occurring Iridoids and Secoiridoids: A Review, Part 2. Chem. Pharm. Bull. (Tokyo) 2007, 55, 689–728.
- Hegnauer, R.; Kooiman, P. The Taxonomic Significance of Iridoids of Tubiflorae Sensu Wettstein (Author’s Transl). Planta Med. 1978, 33, 1–33.
- Sticher, O.V.; Junod-Busch, U. The Iridoid Glucoside and Its Isolation. Pharm. Acta Helvetiae 1975, 50, 127–144.
- Huang, Y.-L.; Oppong, M.B.; Guo, Y.; Wang, L.-Z.; Fang, S.-M.; Deng, Y.-R.; Gao, X.-M. The Oleaceae Family: A Source of Secoiridoids with Multiple Biological Activities. Fitoter 2019, 136, 104155.
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340.
- Somova, L.; Shode, F.; Ramnanan, P.; Nadar, A. Antihypertensive, Antiatherosclerotic and Antioxidant Activity of Triterpenoids Isolated from Olea Europaea, Subspecies Africana Leaves. J. Ethnopharmacol. 2003, 84, 299–305.
- Sheth, A.; Mitaliya, K.D.; Joshi, S. The herbs of Ayurveda, 1st ed.; Ashok Sheth: Gujarat, India, 2005.
- Bellakhdar, J.; Claisse, R.; Fleurentin, J.; Younos, C. Repertory of Standard Herbal Drugs in the Moroccan Pharmacopoea. J. Ethnopharmacol. 1991, 35, 123–143.
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the Anti-Hyperglycemic Effect of Plants Used as Antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110.
- Ribeiro, R.D.A.; De Melo, M.R.; De Barros, F.; Gomes, C.; Trolin, G. Acute Antihypertensive Effect in Conscious Rats Produced by Some Medicinal Plants Used in the State of São Paulo. J. Ethnopharmacol. 1986, 15, 261–269.
- Pieroni, A.; Heimler, D.; Pieters, L.; Van Poel, B.; Vlietinck, A.J. In Vitro Anti-Complementary Activity of Flavonoids from Olive (Olea europaea L.) Leaves. Die Pharm. 1996, 51, 765–768.
- De Feo, V.; Aquino, R.; Menghini, A.; Ramundo, E.; Senatore, F. Traditional Phytotherapy in the Peninsula Sorrentina, Campania, Southern Italy. J. Ethnopharmacol. 1992, 36, 113–125.
- Ghazanfar, S.A.; Al-Al-Sabahi, A.M. Medicinal Plants of Northern and Central Oman (Arabia). Econ. Bot. 1993, 47, 89–98.
- Termentzi, A.; Halabalaki, M.; Skaltsounis, A.L. From Drupes to Olive Oil: An Exploration of Olive Key Metabolites. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; Elsevier: Amsterdam, Netherlands, 2015; pp. 147–177.
- Perez-Martinez, P.; López-Miranda, J.; Blanco-Colio, L.; Bellido, C.; Jimenez, Y.; Moreno, J.A.; Delgado-Lista, J.; Egido, J.; Pérez-Jiménez, F. The Chronic Intake of a Mediterranean Diet Enriched in Virgin Olive Oil, Decreases Nuclear Transcription Factor κB Activation in Peripheral Blood Mononuclear Cells from Healthy Men. Atherosclerosis 2007, 194, e141–e146.
- Jensen, S. Chemotaxonomy of the Oleaceae: Iridoids as Taxonomic Markers. Phytochemistry 2002, 60, 213–231.
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and Biotransformations of Phenol-Conjugated Oleosidic Secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167.
- Maestri, D.; Barrionuevo, D.; Bodoira, R.; Zafra, A.; Jiménez-López, J.; Alché, J.D.D. Nutritional Profile and Nutraceutical Components of Olive (Olea europaea L.) Seeds. J. Food Sci. Technol. 2019, 56, 4359–4370.
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil: An HPLC-Orbitrap-based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Med. 2013, 79, 1576–1587.
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of Malaxation Conditions on Phenol and Volatile Profiles in Olive Paste and the Corresponding Virgin Olive Oils (Olea europaea L. Cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595.
- Caruso, D.; Colombo, R.; Patelli, R.; Giavarini, F.; Galli, G. Rapid Evaluation of Phenolic Component Profile and Analysis of Oleuropein Aglycon in Olive Oil by Atmospheric Pressure Chemical Ionization-Mass Spectrometry (APCI-MS). J. Agric. Food Chem. 2000, 48, 1182–1185.
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.D.R.B., Jr. Chemical Screening of Olive Biophenol Extracts by Hyphenated Liquid Chromatography. Anal. Chim. Acta 2007, 603, 176–189.
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. Metabolites Involved in Oleuropein Accumulation and Degradation in Fruits of Olea europaea L.: Hojiblanca and Arbequina Varieties. J. Agric. Food Chem. 2010, 58, 12924–12933.
- Ryan, D.; Antolovich, M.; Herlt, T.; Prenzler, P.D.; Lavee, S.; Robards, K. Identification of Phenolic Compounds in Tissues of the Novel Olive Cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2002, 50, 6716–6724.
- Ryan, D.; Prenzler, P.D.; Lavee, S.; Antolovich, M. and Robards, K. Quantitative Changes in Phenolic Content during Physiological Development of the Olive (Olea europaea) Cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2003, 51, 2532–2538.
- Savarese, M.; Demarco, E.; Sacchi, R. Characterization of Phenolic Extracts from Olives (Olea europaea cv. Pisciottana) by Electrospray Ionization Mass Spectrometry. Food Chem. 2007, 105, 761–770.
- Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A.; Montedoro, G. High-Performance Liquid Chromatography Evaluation of Phenols in Olive Fruit, Virgin Olive Oil, Vegetation Waters, and Pomace and 1D- and 2D-Nuclear Magnetic Resonance Characterization. J. Am. Oil Chem. Soc. 1999, 76, 873–882.
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 61.
- Dydjow-Bendek, D.; Zagoźdźon, P. Total Dietary Fats, Fatty Acids, and Omega-3/Omega-6 Ratio as Risk Factors of Breast Cancer in the Polish Population—A Case-Control Study. In Vivo 2020, 34, 423–431.
- Wang, J.; Wang, C. Dietary Fat Intake and Risk of Bladder Cancer: Evidence from a Meta-Analysis of Observational Studies. Cell. Mol. Boil. 2019, 65, 5–9.
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean Diet and Cancer: Epidemiological Evidence and Mechanism of Selected Aspects. BMC Surg. 2013, 13, S14.
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-Associated Macrophages (TAM) as Major Players of the Cancer-Related Inflammation. J. Leukoc. Boil. 2009, 86, 1065–1073.
- Hamdi, H.K.; Castellon, R. Oleuropein, A Non-Toxic Olive Iridoid, Is an Anti-Tumor Agent and Cytoskeleton Disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778.
- Notarnicola, M.; Pisanti, S.; Tutino, V.; Bocale, D.; Rotelli, M.T.; Gentile, A.; Memeo, V.; Bifulco, M.; Perri, E.; Caruso, M.G. Effects of Olive Oil Polyphenols on Fatty Acid Synthase Gene Expression and Activity in Human Colorectal Cancer Cells. Genes Nutr. 2011, 6, 63–69.
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; De La Lastra, C.A. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156.
- Hassan, Z.K.; Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; Al-Olayan, E.M.; Elobeid, M.A.; Virk, P.; Mohammed, O.B. Oleuropein Induces Anti-Metastatic Effects in Breast Cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4555–4559.
- Elamin, M.H.; Daghestani, M.H.; Omer, S.A.; A Elobeid, M.; Virk, P.; Al-Olayan, E.M.; Hassan, Z.K.; Mohammed, O.B.; Aboussekhra, A. Olive Oil Oleuropein Has Anti-Breast Cancer Properties with Higher Efficiency on ER-Negative Cells. Food Chem. Toxicol. 2013, 53, 310–316.
- Chimento, A.; Casaburi, I.; Rosano, C.; Avena, P.; De Luca, A.; Campana, C.; Martire, E.; Santolla, M.F.; Maggiolini, M.; Pezzi, V.; et al. Oleuropein and Hydroxytyrosol Activate GPER/ GPR30-Dependent Pathways Leading to Apoptosis of ER-Negative SKBR3 Breast Cancer Cells. Mol. Nutr. Food Res. 2014, 58, 478–489.
- Hassan, Z.K.; Elamin, M.H.; Omer, S.A.; Daghestani, M.H.; Al-Olayan, E.S.; Elobeid, M.A.; Virk, P. Oleuropein Induces Apoptosis via the p53 Pathway in Breast Cancer Cells. Asian Pac. J. Cancer Prev. 2014, 14, 6739–6742.
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein Induces Apoptosis via Abrogating NF-κB Activation Cascade in Estrogen Receptor–Negative Breast Cancer Cells. J. Cell. Biochem. 2018, 120, 4504–4513.
- Choupani, J.; Alivand, M.R.M.; Derakhshan, S.; Zaeifizadeh, M.S.; Khaniani, M. Oleuropein Inhibits Migration Ability Through Suppression of Epithelial-Mesenchymal Transition and Synergistically Enhances Doxorubicin-Mediated Apoptosis in MCF-7 Cells. J. Cell. Physiol. 2019, 234, 9093–9104.
- Bayat, S.; Derakhshan, S.M.; Derakhshan, N.M.; Khaniani, M.S.; Alivand, M.R. Downregulation ofHDAC2andHDAC3via oleuropein as a Potent Prevention and Therapeutic Agent in MCF-7 Breast Cancer Cells. J. Cell. Biochem. 2019, 120, 9172–9180.
- Mansouri, N.; Alivand, M.R.; Bayat, S.; Khaniani, M.S.; Derakhshan, S.M. The Hopeful Anticancer Role of Oleuropein in Breast Cancer through Histone Deacetylase Modulation. J. Cell. Biochem. 2019, 120, 17042–17049.
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anticancer Agents Med. Chem. 2019, 19, 1983–1990.
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B Phosphatase as a Novel Target of Oleuropein Activity in MCF-7 Breast Cancer Model. Toxicol. Vitr. 2019, 61, 104624.
- Yan, C.-M.; Chai, E.-Q.; Cai, H.-Y.; Miao, G.-Y.; Ma, W. Oleuropein Induces Apoptosis via Activation of Caspases and Suppression of Phosphatidylinositol 3-kinase/protein kinase B Pathway in HepG2 Human Hepatoma Cell Line. Mol. Med. Rep. 2015, 11, 4617–4624.
- Sherif, I.O.; Al-Gayyar, M.M. Oleuropein Potentiates Anti-Tumor Activity of Cisplatin Against HepG2 through Affecting proNGF/NGF Balance. Life Sci. 2018, 198, 87–93.
- Vanella, L.; Acquaviva, R.; Di Giacomo, C.; Sorrenti, V.; Galvano, F.; Santangelo, R.; Cardile, V.; Gangia, S.; D’Orazio, N.; Abraham, N.G. Antiproliferative Effect of Oleuropein in Prostate Cell Lines. Int. J. Oncol. 2012, 41, 31–38.
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and Antigrowth Action of Peracetylated Oleuropein in Thyroid Cancer Cells. J. Mol. Endocrinol. 2013, 51, 181–189.
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated with Activation of the c-Jun NH2-Terminal Kinase. J. Pharmacol. Sci. 2014, 125, 300–311.
- Seçme, M.; Eroğlu, C.; Dodurga, Y.; Bağcı, G. Investigation of Anticancer Mechanism of Oleuropein via Cell Cycle and Apoptotic Pathways in SH-SY5Y Neuroblastoma Cells. Gene 2016, 585, 93–99.
- Liu, M.; Wang, J.; Huang, B.; Chen, A.; Li, X. Oleuropein Inhibits the Proliferation and Invasion of Glioma Cells via Suppression of the AKT Signaling Pathway. Oncol. Rep. 2016, 36, 2009–2016.
- Xu, T.; Xiao, D. Oleuropein Enhances Radiation Sensitivity of Nasopharyngeal Carcinoma by Downregulating PDRG1 through HIF1α-repressed microRNA-519d. J. Exp. Clin. Cancer Res. 2017, 36, 3.
- Cao, S.; Zhu, X.; Du, L. P38 MAP Kinase is Involved in Oleuropein-Induced Apoptosis in A549 Cells by a Mitochondrial Apoptotic cascade. Biomed. Pharmacother. 2017, 95, 1425–1435.
- Wang, W.; Wu, J.; Zhang, Q.; Li, X.; Zhu, X.; Wang, Q.; Cao, S.; Du, L. Mitochondria-Mediated Apoptosis Was Induced by Oleuropein in H1299 Cells Involving Activation of p38 MAP Kinase. J. Cell. Biochem. 2019, 120, 5480–5494.
- Antognelli, C.; Frosini, R.; Santolla, M.F.; Peirce, M.J.; Talesa, V.N. Oleuropein-Induced Apoptosis Is Mediated by Mitochondrial Glyoxalase 2 in NSCLC A549 Cells: A Mechanistic Inside and a Possible Novel Nonenzymatic Role for an Ancient Enzyme. Oxidative Med. Cell. Longev. 2019, 2019, 8576961.
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937.
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950.
- Zhang, F.; Zhang, M. Oleuropein Inhibits Esophageal Cancer through Hypoxic Suppression of BTG3 mRNA. Food Funct. 2019, 10, 978–985.
- Przychodzen, P.; Wyszkowska, R.; Gorzynik-Debicka, M.; Kostrzewa, T.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Anticancer Potential of Oleuropein, the Polyphenol of Olive Oil, With 2-Methoxyestradiol, Separately or in Combination, in Human Osteosarcoma Cells. Anticancer. Res. 2019, 39, 1243–1251.
- Barzegar, F.; Zaefizadeh, M.; Yari, R.; Salehzadeh, A. Synthesis of Nano-Paramagnetic Oleuropein to Induce KRAS Over-Expression: A New Mechanism to Inhibit AGS Cancer Cells. Medicina (Kaunas) 2019, 55, E388.
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein Aglycone Induces Autophagy via the AMPK/mTOR Signalling Pathway: A Mechanistic Insight. Oncotarget 2015, 6, 35344–35357.
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a Phenolic Compound of Virgin Olive Oil, Activates AMP-Activated Protein Kinase to Inhibit Carcinogenesis. Carcinogenesis 2011, 32, 545–553.
- Elnagar, A.; Sylvester, P.; El Sayed, K. (−)-Oleocanthal as a c-Met Inhibitor for the Control of Metastatic Breast and Prostate Cancers. Planta Medica 2011, 77, 1013–1019.
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.-Y.; Sayed, K.A.E. Olive Phenolics as c-Met Inhibitors: (-)-Oleocanthal Attenuates Cell Proliferation, Invasiveness, and Tumor Growth in Breast Cancer Models. PLOS ONE 2014, 9, e97622.
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; Sayed, K.A. El Olive Oil-derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies. Phyther. Res. 2015, 29, 1776–1782.
- Tajmim, A.; Siddique, A.B.; Sayed, E.; El Sayed, K. Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities. Pharmaceutics 2019, 11, E515.
- Qusa, M.H.; Siddique, A.B.; Nazzal, S.; El Sayed, K.A. Novel Olive Oil Phenolic (−)-Oleocanthal (+)-Xylitol-Based Solid Dispersion Formulations with Potent Oral Anti-Breast Cancer Activities. Int. J. Pharm. 2019, 569, 118596.
- Siddique, A.B.; Ebrahim, H.Y.; Akl, M.R.; Ayoub, N.M.; Goda, A.A.; Mohyeldin, M.M.; Nagumalli, S.K.; Hananeh, W.M.; Liu, Y.-Y.; Meyer, S.A.; et al. (−)-Oleocanthal Combined with Lapatinib Treatment Synergized against HER-2 Positive Breast Cancer in Vitro and in Vivo. Nutrients 2019, 11, 412.
- Diez-Bello, R.; Jardin, I.; Lopez, J.J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.M.; Salido, S.; Rosado, J.A. (−)-Oleocanthal Inhibits Proliferation and Migration by Modulating Ca2+ Entry through TRPC6 in Breast Cancer Cells. Biochim. Biophys. Acta - Mol. Cell Res. 2019, 1866, 474–485.
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal Inhibits Growth and Metastasis by Blocking Activation of STAT3 in Human Hepatocellular Carcinoma. Oncotarget 2016, 7, 43475–43491.
- De Stefanis, D.; Scimè, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P.; Stefanis, D. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 11, 1640.
- Margarucci, L.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Monti, M.C.; Tosco, A.; Casapullo, A. Chemical Proteomics-Driven Discovery of Oleocanthal as an Hsp90 Inhibitor. Chem. Commun. 2013, 49, 5844.
- Gu, Y.; Wang, J.; Peng, L. (−)-Oleocanthal Exerts Anti-Melanoma Activities and Inhibits STAT3 Signaling Pathway. Oncol. Rep. 2017, 37, 483–491.
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and Oleacein Contribute to the in Vitro Therapeutic Potential of in Oil-Derived Extracts in Non-Melanoma Skin Cancer. Toxicol. Vitr. 2018, 52, 243–250.
- Fabiani, R.; Rosignoli, P.; De Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA Damage Is Prevented by Extracts of Olive Oil, Hydroxytyrosol, and Other Olive Phenolic Compounds in Human Blood Mononuclear Cells and HL60 Cells. J. Nutr. 2008, 138, 1411–1416.
- Juli, G.; Oliverio, M.; Bellizzi, D.; Cantafio, M.E.G.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990.
- Kimura, Y.; Sumiyoshi, M. Olive Leaf Extract and Its Main Component Oleuropein Prevent Chronic Ultraviolet B Radiation-Induced Skin Damage and Carcinogenesis in Hairless Mice. J. Nutr. 2009, 139, 2079–2086.
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive Effect of Oleuropein in Colitis-Associated Colorectal Cancer in c57bl/6 Mice. Mol. Nutr. Food Res. 2016, 60, 242–255.
- Geyikoğlu, F.; Çolak, S.; Türkez, H.; Bakır, M.; Koç, K.; Hosseinigouzdagani, M.K.; Çeriğ, S.; Sönmez, M. Oleuropein Ameliorates Cisplatin-induced Hematological Damages Via Restraining Oxidative Stress and DNA Injury. Indian J. Hematol. Blood Transfus. 2017, 33, 348–354.
- Song, H.; Lim, Y.; Jung, J.I.; Cho, H.J.; Park, S.Y.; Kwon, G.T.; Kang, Y.-H.; Lee, K.W.; Choi, M.-S.; Park, J.H.Y. Dietary Oleuropein Inhibits Tumor Angiogenesis and Lymphangiogenesis in the B16F10 Melanoma Allograft Model: A Mechanism for the Suppression of High-Fat Diet-Induced Solid Tumor Growth and Lymph Node Metastasis. Oncotarget 2017, 8, 32027–32042.
- Koc, K.; Bakir, M.; Geyikoglu, F.; Cerig, S. Therapeutic Effects of Oleuropein on Cisplatin-Induced Pancreas Injury in Rats. J. Cancer Res. Ther. 2018, 14, 671.
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.H.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; Elkhadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment with Oleuropein and Doxorubicin in Vivo. Altern. Ther. Health Med. 2019, 25, 17–24.
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Locoregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637.
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; A Bhutta, Z.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of Phenolic Extracts from Brava Extra Virgin Olive Oils and Their Cytotoxic Effects on MCF-7 Breast Cancer Cells. Food Chem. Toxicol. 2018, 119, 73–85.
- Crespo, M.C.; Tomé-Carneiro, J.; Dávalos, A.; Visioli, F. Pharma-Nutritional Properties of Olive Oil Phenols. Transfer of New Findings to Human Nutrition. Foods 2018, 7, 90.
- Catalán, Ú.; Rubio, L.; Hazas, M.-C.L.D.L.; Herrero, P.; Nadal, P.; Canela, N.; Pedret, A.; Motilva, M.-J.; Solà, R. Hydroxytyrosol and Its Complex Forms (Secoiridoids) Modulate Aorta and Heart Proteome in Healthy Rats: Potential Cardio-Protective Effects. Mol. Nutr. Food Res. 2016, 60, 2114–2129.
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low Density Lipoprotein Oxidation Is Inhibited in Vitro by Olive Oil Constituents. Atherosclerosis 1995, 117, 25–32.
- Visioli, F.; Bellosta, S.; Galli, C. Oleuropein, the Bitter Principle of Olives, Enhances Nitric Oxide Production by Mouse Macrophages. Life Sci. 1998, 62, 541–546.
- Parzonko, A.; Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and Oleacein may Restore Biological Functions of Endothelial Progenitor Cells Impaired by Angiotensin II via Activation of Nrf2/heme Oxygenase-1 Pathway. Phytomedicine 2013, 20, 1088–1094.
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E.; et al. Minor Components of Olive Oil Modulate Proatherogenic Adhesion Molecules Involved in Endothelial Activation. J. Agric. Food Chem. 2006, 54, 3259–3264.
- Leri, M.; Nosi, D.; Natalello, A.; Porcari, R.; Ramazzotti, M.; Chiti, F.; Bellotti, V.; Doglia, S.M.; Stefani, M.; Bucciantini, M. The Polyphenol Oleuropein Aglycone Hinders the Growth of Toxic Transthyretin Amyloid Assemblies. J. Nutr. Biochem. 2016, 30, 153–166.
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A Comparison of Antioxidant Activities of Oleuropein and Its Dialdehydic Derivative from Olive Oil, Oleacein. Food Chem. 2012, 131, 940–947.
- Czerwińska, M.E.; Kiss, A.K.; Naruszewicz, M. Inhibition of Human Neutrophils NEP Activity, CD11b/CD18 Expression and Elastase Release by 3,4-dihydroxyphenylethanol-elenolic Acid Dialdehyde, Oleacein. Food Chem. 2014, 153, 1–8.
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Wrzosek, M.; Naruszewicz, M. Oleacein Enhances Anti-Inflammatory Activity of Human Macrophages by Increasing CD163 Receptor Expression. Phytomedicine 2015, 22, 1255–1261.
- Manna, C.; Migliardi, V.; Golino, P.; Scognamiglio, A.; Galletti, P.; Chiariello, M.; Zappia, V. Oleuropein Prevents Oxidative Myocardial Injury Induced by Ischemia and Reperfusion. J. Nutr. Biochem. 2004, 15, 461–466.
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Constantinou, M.; Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Kamber, E.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. The Olive Constituent Oleuropein Exhibits Anti-Ischemic, Antioxidative, and Hypolipidemic Effects in Anesthetized Rabbits. J. Nutr. 2006, 136, 2213–2219.
- Andreadou, I.; Sigala, F.; Iliodromitis, E.K.; Papaefthimiou, M.; Sigalas, C.; Aligiannis, N.; Savvari, P.; Gorgoulis, V.; Papalabros, E.; Kremastinos, D.T. Acute Doxorubicin Cardiotoxicity Is Successfully Treated with the Phytochemical Oleuropein through Suppression of Oxidative and Nitrosative Stress. J. Mol. Cell. Cardiol. 2007, 42, 549–558.
- Andreadou, I.; Papaefthimiou, M.; Zira, A.; Constantinou, M.; Sigala, F.; Skaltsounis, A.-L.; Tsantili-Kakoulidou, A.; Iliodromitis, E.K.; Kremastinos, D.T.; Mikros, E. Metabonomic Identification of Novel Biomarkers in Doxorubicin Cardiotoxicity and Protective Effect of the Natural Antioxidant Oleuropein. NMR Biomed. 2009, 22, 585–592.
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.-I.; et al. Oleuropein Prevents Doxorubicin-Induced Cardiomyopathy Interfering with Signaling Molecules and Cardiomyocyte Metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16.
- Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; Yoshimura, H.; Zhong, L. The Anti-Atherosclerotic Effect of Olive Leaf Extract Is Related to Suppressed Inflammatory Response in Rabbits with Experimental Atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243.
- Ebaid, G.M.; Seiva, F.R.; Rocha, K.K.; A Souza, G.; Novelli, E.L. Effects of Olive Oil and Its Minor Phenolic Constituents on Obesity-Induced Cardiac Metabolic Changes. Nutr. J. 2010, 9, 46.
- Khalili, A.; Nekooeian, A.A.; Khosravi, M.B. Oleuropein Improves Glucose Tolerance and Lipid Profile in Rats with Simultaneous Renovascular Hypertension and Type 2 Diabetes. J. Asian Nat. Prod. Res. 2017, 19, 1011–1021.
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Oleuropein Offers Cardioprotection in Rats with Simultaneous Type 2 Diabetes and Renal Hypertension. Indian J. Pharmacol. 2014, 46, 398–403.
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Effects of Oleuropein in Rats with Simultaneous Type 2 Diabetes and Renal Hypertension: A Study of Antihypertensive Mechanisms. J. Asian Nat. Prod. Res. 2014, 16, 953–962.
- Ivanov, M.; Vajic, U.-J.; Mihailovic-Stanojevic, N.; Miloradovic, Z.; Jovovic, D.; Grujic-Milanovic, J.; Karanovic, D.; Dekanski, D. Highly Potent Antioxidant Olea europaea L. Leaf Extract Affects Carotid and Renal Haemodynamics in Experimental Hypertension: The Role of Oleuropein. Excli J. 2018, 17, 29–44.
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and Antioxidant Activities of Oleuropein and Its Hydrolysis Derivative-Rich Extracts from Chemlali Olive Leaves. Chem. Interactions 2008, 176, 88–98.
- Miceli, C.; Santin, Y.; Manzella, N.; Coppini, R.; Berti, A.; Stefani, M.; Parini, A.; Mialet-Perez, J.; Nediani, C. Oleuropein Aglycone Protects against MAO-A-Induced Autophagy Impairment and Cardiomyocyte Death through Activation of TFEB. Oxidative Med. Cell. Longev. 2018, 2018, 8067592.
- Margheri, F.; Menicacci, B.; Laurenzana, A.; Del Rosso, M.; Fibbi, G.; Cipolleschi, M.G.; Ruzzolini, J.; Nediani, C.; Mocali, A.; Giovannelli, L. Oleuropein Aglycone Attenuates the Pro-Angiogenic Phenotype of Senescent Fibroblasts: A Functional Study in Endothelial Cells. J. Funct. Foods 2019, 53, 219–226.
- Covas, M.-I.; Nyyssönen, K.; E Poulsen, H.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; De La Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 333–341.
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) Leaf Extract Effective in Patients with Stage-1 Hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258.
- Stock, J. HDL Function and Cardiovascular Risk: Debate Continues…. Atherosclerosis 2013, 229, 38–41.
- Casapullo, A.; Del Gaudio, F.; Capolupo, A.; Cassiano, C.; Chiara Monti, M. Multi-Target Profile of Oleocanthal, An Extra-Virgin Olive Oil Component. Curr. Bioact. Compd. 2016, 12, 3–9.
- Montoya, T.; Castejón, M.L.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; De-La-Lastra, C.A. Oleocanthal Modulates LPS-Induced Murine Peritoneal Macrophages Activation via Regulation of Inflammasome, Nrf-2/HO-1, and MAPKs Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5552–5559.
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-Like activIty in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46.
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell. Physiol. Biochem. 2018, 49, 2414–2426.
- Vinten-Johansen, J. Involvement of Neutrophils in the Pathogenesis of Lethal Myocardial Reperfusion Injury. Cardiovasc. Res. 2004, 61, 481–497.
- Filipek, A.; Czerwińska, M.E.; Kiss, A.K.; Polański, J.A.; Naruszewicz, M. Oleacein may Inhibit Destabilization of Carotid Plaques from Hypertensive Patients: Impact on high Mobility Group Protein-1. Phytomedicine 2017, 32, 68–73.
- Paiva-Martins, F.; Fernandes, J.; Santos, V.; Silva, L.; Borges, F.; Rocha, S.; Belo, L.; Santos-Silva, A. Powerful Protective Role of 3,4-dihydroxyphenylethanol-elenolic acid Dialdehyde against Erythrocyte Oxidative-lnduced Hemolysis. J. Agric. Food Chem. 2010, 58, 135–140.
- Naruszewicz, M.; Czerwinska, M.; Kiss, A. Oleacein. Translation from Mediterranean Diet to Potential Antiatherosclerotic Drug. Curr. Pharm. Des. 2014, 21, 1205–1212.
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxidative Med. Cell. Longev. 2016, 2016, 1–15.
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230.
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186.
- Stefani, M.; Rigacci, S. Beneficial Properties of Natural Phenols: Highlight on Protection against Pathological Conditions Associated with Amyloid Aggregation. BioFactors 2014, 40, 482–493.
- Capurso, A.; Crepaldi, G.; Capurso, C. Extra-Virgin Olive Oil, the Mediterranean Diet, and Neurodegenerative Diseases. In Benefits of the Mediterranean Diet in the Elderly Patient; Springer: Cham, Switzerland, 2018; pp. 81–95.
- Pasban-Aliabadi, H.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M.; Mehdizadeh, A.; Yaghoobi, M.M. Inhibition of 6-Hydroxydopamine-Induced PC12 Cell Apoptosis by Olive (Olea europaea L.) Leaf Extract Is Performed by Its Main Component Oleuropein. Rejuvenation Res. 2013, 16, 134–142.
- Achour, I.; Arel-Dubeau, A.-M.; Renaud, J.; Legrand, M.; Attard, E.; Germain, M.; Martinoli, M.-G. Oleuropein Prevents Neuronal Death, Mitigates Mitochondrial Superoxide Production and Modulates Autophagy in a Dopaminergic Cellular Model. Int. J. Mol. Sci. 2016, 17, 1293.
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuropein aglycone and hydroxytyrosol interfere differently with toxic Aβ1-42 aggregation. Food Chem. Toxicol. 2019, 129, 1–12.
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. Int. J. Mol. Sci. 2019, 20, E125.
- Luccarini, I.; Dami, T.E.; Grossi, C.; Rigacci, S.; Stefani, M.; Casamenti, F. Oleuropein Aglycone Counteracts Aβ42 Toxicity in the Rat Brain. Neurosci. Lett. 2014, 558, 67–72.
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Dami, T.E.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The Polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß Plaque Pathology. PLOS ONE 2013, 8, e71702.
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein Aglycone Protects Transgenic C. elegans Strains Expressing Aβ42 by Reducing Plaque Load and Motor Deficit. PLOS ONE 2013, 8, e58893.
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Nichino, D.; Relini, A.; Berti, A.; Stefani, M. Aβ(1-42) Aggregates into Non-Toxic Amyloid Assemblies in the Presence of the Natural Polyphenol Oleuropein Aglycon. Curr. Alzheimer Res. 2011, 8, 841–852.
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein Aglycone and Polyphenols from Olive Mill Waste Water Ameliorate Cognitive Deficits and Neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62.
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-Rich Extra-Virgin Olive Oil Enhances Donepezil Effect by Reducing Amyloid-β Load and Related Toxicity in a Mouse Model of Alzheimer’s Disease. J. Nutr. Biochem. 2018, 55, 113–123.
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849–1859.
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-Oil-Derived Oleocanthal Enhances β-Amyloid Clearance as a Potential Neuroprotective Mechanism against Alzheimer’s Disease: In Vitro and in Vivo Studies. ACS Chem. Neurosci. 2013, 4, 973–982.
- Mohammad-Beigi, H.; Aliakbari, F.; Sahin, C.; Lomax, C.; Tawfike, A.; Schafer, N.P.; Amiri-Nowdijeh, A.; Eskandari, H.; Møller, I.M.; Hosseini-Mazinani, M.; et al. Oleuropein Derivatives from Olive Fruit Extracts Reduce α-Synuclein Fibrillation and Oligomer Toxicity. J. Boil. Chem. 2019, 294, 4215–4232.




