Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali Mirzaei | + 3045 word(s) | 3045 | 2021-06-08 10:47:44 | | | |
| 2 | Camila Xu | Meta information modification | 3045 | 2021-06-23 10:27:30 | | | | |
| 3 | Camila Xu | Meta information modification | 3045 | 2021-06-23 10:28:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mirzaei, A. CuxO Nanostructure-Based Gas Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/11180 (accessed on 07 February 2026).
Mirzaei A. CuxO Nanostructure-Based Gas Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/11180. Accessed February 07, 2026.
Mirzaei, Ali. "CuxO Nanostructure-Based Gas Sensors" Encyclopedia, https://encyclopedia.pub/entry/11180 (accessed February 07, 2026).
Mirzaei, A. (2021, June 23). CuxO Nanostructure-Based Gas Sensors. In Encyclopedia. https://encyclopedia.pub/entry/11180
Mirzaei, Ali. "CuxO Nanostructure-Based Gas Sensors." Encyclopedia. Web. 23 June, 2021.
Copy Citation
H2S gas is a toxic and hazardous byproduct of the oil and gas industries. It paralyzes the olfactory nerves, with concentrations above 100 ppm, resulting in loss of smell; prolonged inhalation may even cause death. One of the most important semiconducting metal oxides for the detection of H2S is CuxO (x = 1, 2), which is converted to CuxS upon exposure to H2S, leading to a remarkable modulation in the resistance and appearance of an electrical sensing signal.
gas sensor
HS gas
CuO
CuS
sensing mechanism
1. Overview of H2S Gas Properties
H2S, which has a disagreeable odor, is a colorless, highly toxic, corrosive, flammable, and explosive gas [1][2]. It has various sources, including volcanic eruptions, anaerobic decay of organic materials, Kraft paper mills, processing of gasoline, natural gas, coal, and sewage, as well as petroleum refining [3][4][5][6]. Other sources of H2S include decomposition of fish in the fishing industry and waste biodegradation in shrimp farms [7]. H2S is readily oxidized by atmospheric oxygen when irradiated with sunlight and can form SO2 and H2SO4 in the atmosphere, leading to acid rains [8]. Moreover, because H2S is heavier than air, it remains in the atmosphere for up to 18 h; thus, it can easily accumulate in areas such as mine tunnels, sewers, and manure tanks [9][10]. As stated by the United States Occupational Safety and Health Administration (US-OSHA, Washington, DC, USA), the concentration limit for exposure to H2S within 8 h is approximately 20 ppm [11]. Exposure to low levels of H2S may result in eye and throat injuries, dizziness, memory impairment, and loss of reasoning and balance [12][13][14][15]. Continuous exposure to H2S at 2 ppm causes nausea/headaches, while 20 ppm results in fatigue and headache, and concentrations of 50–100 ppm result in loss of appetite, respiratory tract irritation, digestion issues, and loss of breathing with high possibility of death [13][16]. Moreover, an individual’s olfaction may quickly fail to function properly when exposed to H2S levels of more than 100 ppm for a short period of time [17]. Prolonged exposure to 100 ppb of H2S also affects respiration in human cells and causes a deficiency of oxygen in systemic tissues, endangering human health [18][19]. H2S exposure at concentrations of 1000 ppm and above would result in immediate death. For example, 21 fatalities were reported in the Gulf of Mexico in 2007 as a result of H2S leaks and chain accidents on the Kab-121 platform [20]. In addition, H2S is a known biomarker for various diseases such as Down syndrome, Alzheimer’s disease, ischemia, asthma, and halitosis [21]. For example, the H2S concentration (~100–500 ppb) in the breath of patients with halitosis is greater than that in healthy individuals. This is primarily due to local oral diseases and certain systemic diseases, such as digestive issues [22]. Accordingly, detection of H2S gas is important from the perspectives of safety, industry, and medicine.
2. Motivation for the Use of CuxO as a H2S Gas Sensor
To date, several types of gas sensors, including chemoresistive gas sensors made of semiconducting metal oxides, electrochemical sensors, optical sensors, and piezoelectric sensors, have been utilized for H2S gas detection [23][24][25]. Among them, semiconducting metal oxide gas sensors are widely popular owing to their advantages such as simple operation, small size, low cost, high sensitivity, high stability, fast response, and long life [26][27]. Overall, the sensing mechanism in this type of gas sensor is the change in resistance caused by exposure to target gases [28][29]. The gas response depends mainly on the contact between the target gases and the surface of the sensing element, as well as the adsorption sites on the sensor surface [30]. Among the different metal oxides used for H2S-sensing, CuxO (x = 1, 2) is an exception. CuO is a low-cost semiconducting metal oxide (energy gap = 1.2 eV) and has a monoclinic crystal structure [31]. It can directly react with H2S to form a CuxS layer with high conductivity. Primarily, CuS forms clusters on the surface of CuO, which grows progressively and links to create a persistent CuS phase with metallic-like conductivity. This process generates a conductive percolation pathway, which consequently decreases the resistance abruptly [32]. The electrons flow from CuO to CuS owing to the difference in their work functions and formation of a potential barrier. When the grain boundaries transform into CuS, the width of the potential barrier is minimized, and the conductivity changes from semiconducting to metallic, resulting in an advanced H2S response [33]. Thus, CuxO is highly popular for H2S gas-sensing studies [12][26][34]. Furthermore, it can be used as an adsorbent for the purification of gas streams, especially by H2S removal [35]. In addition, the selectivity of CuxO can be related to the lower bond energy of H2S relative to other gases. As shown in Table 1, the H‒SH bonding energy in H2S is 381 kJ/mol, which is lower than that of most interfering gases, leading to better interaction and dissociation of the surface of the sensing material.
Table 1. Various gas molecules and their properties [36].
| Gas | Ammonia (NH3) | Hydrogen (H2) | Methane (CH4) | Carbon Monoxide (CO) | H2S |
|---|---|---|---|---|---|
| Bond | H‒NH2 | H‒H | H‒CH3 | C‒O | H‒SH |
| Bond Energy (kJ/mol) | 435 | 436 | 431 | 1076 | 381 |
| Molecular Size (nm) | 0.26 | 0.289 | 0.38 | 0.37 | 0.34 |
3. Gas Sensors Based on Pristine CuxO Nanostructures
Hu et al. [37] conducted a study on the magnetron sputtering of p-type CuO nanoneedle arrays followed by wet chemical etching and annealing. Their CuO sensor demonstrated response of 76.5% (ΔR/Ra) × 100 to 15 ppm H2S gas at 150 °C. The oxygen molecules from air are adsorbed onto the sensor surface to form a hole accumulation layer (HAL); thereafter, the sensor resistance decreases relative to vacuum because of the adsorption of electrons as follows [37]:
O2 (gas) → O2 (ads.),
O2 (ads.) + e− → O2− (ads.),
O2− (ads.) + e− → 2O− (ads.).
When the sensor is in an H2S atmosphere, the chemical reaction between the adsorbed H2S molecules on the sensor surface and the chemisorbed oxygen species occurs as follows:
H2S + 3O− (ads.) → H2O + SO2 + 3e−.
The released electrons are combined with holes, and the thickness of the HAL decreases. Thus, the resistance increases, which leads to the appearance of a sensing signal. However, the authors did not report the formation of CuS, and the selectivity of the gas sensor was not explained. In another study, Huang et al. synthesized sea anemone-like CuO nanoarrays in situ via a seed-induced hydrothermal method at different time durations (1–6 h) [38]. For growth times exceeding 2 h, they observed that the CuO nanostructures grew among the electrodes, and CuO nanostructures and sea anemone array morphologies were formed. The surface areas of CuO nanoarrays prepared for 2, 3, 4, 5, and 6 h were 5.85, 7.35, 7.03, 6.67, and 5.70 m2·g−1, respectively. The surface area decreased with increasing growth time (>2 h). The results of H2S gas-sensing showed that the CuO prepared for 2 h had the lowest response among the other sensors because of the fragile continuity of the tiny CuO nanoarrays, which did not offer the active electrical conduction pathways necessary for the H2S-sensing reactions. The sensor with the largest surface area (prepared for 3 h) showed not only the highest response to H2S gas (24.08 to 5 ppb H2S gas at 25 °C), but also good selectivity.
The HAL was formed in air, and the oxygen adsorption made it wider at the junctions, which enabled hole transmission and subsequently decreased the resistance. In the H2S gas atmosphere, the HAL was narrowed, which hampered the transmission of holes while the sensor resistance increased. In addition, the cross-linked assembly enabled the adsorption, dispersion, and channeling of H2S molecules in detecting reactions and efficiently evaded the lateral stacking and accumulation of CuO nanostructures with no specific surface area loss because of the slight contact and appropriate clearance. This eventually led to a high sensitivity of the gas sensor toward H2S gas.
As humidity is always present in real environments, the development of gas sensors that can operate under humid conditions is important. Accordingly, Miao et al. [39] described the humidity-independent H2S-sensing performance of hydrothermally processed monolayered CuO nanosheet films under dry and wet conditions. Figure 1a,b compares the sensing mechanism of the CuO gas sensor to H2S in dry and humid environments. In particular, in the humid state, previously adsorbed oxygen anions are substituted partly via terminal hydroxyl groups, which results in low reactions between H2S molecules and chemisorbed oxygen atoms. The extent of this limit was determined on the basis of the humidity level in the gas chamber. Accordingly, the sensor signal to H2S decreased steadily as the humidity level increased. However, in H2S-sensing, the inclusion of S in the CuO framework was assisted by the terminal hydroxyl group at the surface. In addition, the higher humidity level resulted in an abundance of terminal hydroxyl groups, and, as a result, greater conversion from CuO to Cu2S and S occurred. This was due to variable oxidation states of Cu and was demonstrated by XPS and SEM studies. Thus, the sensor response was maintained at the same level as the humidity increased. The decreased recovery time observed under humid conditions was elucidated through the low thermal stability of Cu2S. The characteristic Gibbs free energies for the development for CuO, Cu2O, and Cu2S were −129.7 kJ/mol, −146.0 kJ/mol, and −86.2 kJ/mol, respectively. In this way, changing from a less thermodynamically steady phase (i.e., Cu2S) into CuO would be a much more straightforward process than from a steady phase (Cu2O); accordingly, a quicker recovery time was observed under humid conditions [39].
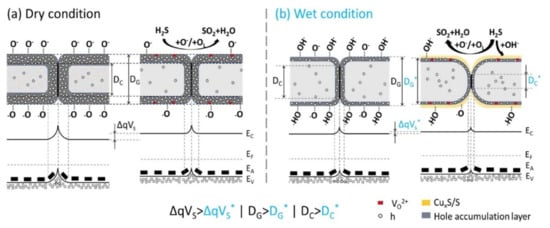
Figure 1. Schematic of H2S detecting mechanism of p-CuO nanosheet gas sensor in (a) dry and (b) humid environments. Reprinted from [39] with permission from Elsevier.
Dhakshinamoorthy et al. [40] described the H2S gas-sensing features of CuO nanocuboids. The fabricated CuO nanocuboid sensor had a response of 2.5 (ΔR/Ra) to 10 ppm H2S at an optimal temperature of 200 °C. When H2S molecules are adsorbed onto the CuO (111) plane, the ‘S’ atom can be bonded to two-fold subsurface Cu atom (Cusub) atoms through the bridge bond. However, the ‘H’ adsorbs favorably on the outermost oxygen (Osuf) site of the CuO lattice and displays good catalytic activity toward H2S dissociation. A comparison of the H2S gas-sensing performance of the pristine CuxO gas sensors is shown in Table 2. Different morphologies have been used for H2S-sensing at different temperatures. However, pristine CuxO gas sensors show lower sensitivity compared to noble metal-decorated gas sensors or composite gas sensors. Therefore, strategies are needed to enhance the response of gas sensors toward H2S gas.
Table 2. H2S gas-sensing properties of pristine CuxO-based gas sensors.
| Sensor | H2S Conc. (ppm) |
Response (Rg/Ra) |
LOD † (ppm) |
Res. Time (s)/ Rec. Time (s) |
T (°C) |
Ref. |
|---|---|---|---|---|---|---|
| CuO nanoparticles | 5 | 4.9 ± 0.43 | 0.2 | 297.5 ± 9.2/54 ± 7.1 | 40 | [12] |
| CuO nanoneedles | 10 | 76.5% (ΔR/Ra) × 100 | 161 ppb | 92/196 | 150 | [37] |
| Sea anemone-like CuO nanoarrays | 5 ppb | 24.08 | 1.52 ppb | 102/539 | 25 | [38] |
| CuO nanosheets | 400 ppb | 1.7 | 3 ppb | NA * | 325 | [39] |
| CuO nanocuboids | 10 | ~2.4 (ΔR/Ra) | 1 | NA | 200 | [40] |
| CuO nanosheets | 1 | 325% [(ΔR/Ra) × 100] | 2 ppb | 4/9 | 240 | [41] |
| CuO nanowires | 100 ppb | ~0.2 (ΔR/Ra) | 2.5 ppb | 10 min/15 min | 180 | [42] |
* NA: not available; † LOD: limit of detection; T = operating temperature
4. Pd-Decorated/Doped CuxO Nanostructure-Based Gas Sensors
To boost the sensing performance of metal oxide gas sensors, the addition of noble metals such as Pd, Pt, and Au has been studied extensively because of their electronic and chemical sensitization properties [43][44][45][46][47]. Unfortunately, there are very few studies related to the noble metal decoration on the surface of CuxO nanostructures, and these are discussed below. Kim et al. [27] improved the H2S-sensing performance of CuO nanowires (NWs) in self-heating mode through Pd decoration. They used a thermal oxidation method to grow network-like CuO NWs on a patterned interdigital electrode on a SiO2-grown Si substrate, and subsequent Pd decoration was achieved through UV irradiation. Their sensing results corroborate that the sensor made of pristine CuO NWs did not show H2S selectivity at an optimum temperature of 300 °C. However, the Pd-functionalized CuO NW sensor exhibited a higher response to H2S at 100 °C. In addition, the pristine and Pd-loaded sensors showed responses (Rg/Ra) of 1.08 and 1.89, respectively, to 100 ppm H2S in the self-heating mode at 5 V. The generated heat was due to the self-heating effects attributable to (i) the electron current passing through the CuO NWs, and (ii) the number of networked and directly connected CuO NWs. Because of chemical sensitization, Pd may simply dissociate and transfer oxygen molecules and target gases via the spillover effect. Consequently, higher quantities of gas molecules can reach the surface of the sensor, and, accordingly, a better response is expected. In addition, some Pd can be partially converted into PdO in the air. The electrons flow from CuO to Pd/PdO because of the smaller work function of CuO compared to Pd and PdO. Therefore, an HAL is formed on the CuO side. Upon reducing the gas exposure, the width of the HAL in CuO is condensed. As the initial volume/concentration of holes increases due to the presence of Pd/PdO, the reduction in the same number of holes due to exposure to the reducing gas results in a low response of the sensor. This results in a decrease in the sensor response to the reducing gases. However, the situation is different for H2S-sensing. CuO is transformed into CuS with metal-like conductivity upon exposure to H2S, which results in a high resistance modulation at the heterojunctions. The height of the potential barriers decreases owing to the different work functions of CuO and CuS. This results in a higher concentration of electrons in the sensing layer (CuO/CuS) than in CuO, which decreases the concentration of holes and increases the resistance of the sensing material. In the p-type sensor, a smaller conduction volume of the hole region delivers a high sensor response.
In another study, Kim et al. [48] described the development of CuO nanorods (NRs) and their respective Pd-functionalization via a three-step approach including Cu foil oxidation in air, dipping in PdCl2, and subsequent annealing. In air, the CuO surface adsorbs oxygen from air by capturing electrons, resulting in the formation of HAL. Upon exposure to low concentrations of H2S, the surface reactions occur spontaneously between the formerly adsorbed oxygen species and H2S molecules; subsequently, the electrons released from the surface states recombine with the holes in the valence band, leading to an increase in the electrical resistance of the gas sensor. In contrast, at high H2S concentrations, a layer of CuS is created on the CuO NR surface. The configuration of CuS reduces the resistance to its metallic character. In the case of Pd/CuO NRs, the adsorption and generation of free electrons through H2S gas are advanced on the surface of Pd nanoparticles (NPs). The Pd-NPs have a larger surface area than the CuO NRs, leading to better H2S adsorption. The H2S molecules dissociate on the catalytic Pd-clusters and diffuse, possibly across and/or through the clusters, to the substrate, wherein H2S gas molecules may correlate with the CuO NRs to enhance the resistance. Therefore, through Pd loading, a higher response was observed.
Mikami et al. [49], reported the synthesis of Pd-loaded Cu2O nanocrystals, which corroborated the H2S responses at low concentrations (1‒8 ppm) between 50 °C and 150 °C. The electrical resistance of Cu2O was found to increase upon Pd loading because of the configuration of the Schottky junctions between Cu2O and Pd. They noticed that the resistance of the sensor decreased abruptly for high Pd loading (5 mol.% and 10 mol.%), signifying that adding a suitable quantity of Pd to the Cu2O nanocrystals suppresses Cu2O sulfurization and supports the reaction of the adsorbed oxygen with H2S. In fact, catalytic H2S dissociation on Pd facilitated the reaction of adsorbed oxygen with H2S gas. Upon stoppage of H2S gas, Cu2O and CuO oxides were formed in the air according to the following equations:
Cu2S + 3/2O2 → Cu2O + SO2,
CuS + 3/2O2 → CuO + SO2.
The standard Gibbs free energies of Equations (5) and (6) at 50 °C and 150 °C are −359.411, −372.825, −350.166, and −364.33 kJ/mol, respectively, indicating that these reactions are thermodynamically favorable at 50 and 150 °C. This demonstrates the reversible nature of gas sensors.
Lastly, Hu et al. [50] developed a Pd-doped (0‒1.5 wt.%) CuO gas sensor for H2S-sensing. The ionic radius of Cu2+ (0.73 Å) is smaller than that of Pd2+ (0.86 Å), and the replacement of Cu2+ through Pd2+ ions induced lattice growth, which was supported by the XRD pattern. Sensing studies showed that the H2S response of the Pd-doped CuO (1.25 wt.%) sensor was 7.9 times greater than that of the pristine CuO sensor (Rg/Ra = 15.7) at 80 °C. The upgraded response characteristics of the Pd-doped CuO were largely ascribed to electronic sensitization. Even at room temperature, PdO has a high intrinsic carrier concentration and conductivity; thus, Pd doping can decrease the working temperature of the Pd/CuO sensor. Additionally, PdO is capable of boosting the sensing material to capture oxygen molecules and form chemisorbed oxygen atoms. In addition, due to the sensitization, PdO molecules on the surface of CuO can take part in the reactions and release electrons once they are exposed to H2S. Accordingly, the released electrons can be recombined with holes, resulting in a reduction in the concentration of holes. Thereafter, the resistance of the Pd-doped CuO sensor increased with contact with H2S. Compared to pristine CuO, Pd-doped CuO (1.25 wt.%) had a superior surface area and high density of adsorption sites, which resulted in excellent responses even at comparatively low H2S concentrations. However, surface disorder occurred as the doping concentration increased to 1.50 wt.%, and it was accompanied by an increase in the density of surface states, leading to the pinning of Fermi level surfaces and, hence, a decrease in the sensor response. A list of noble-metal-decorated/doped CuO-based gas sensors for H2S detection is presented in Table 3. As can be seen, only Pd has been used in combination with CuxO; in this regard, decoration/doping with other noble metals is essential. In general, Pd-decoration/doping on CuO can enhance the response of gas sensors relative to pristine gas sensors. Furthermore, it can decrease the sensing temperature and increase the stability of gas sensors [27][48][49][50].
Table 3. H2S gas-sensing properties of noble metal-decorated/doped CuO-based gas sensors.
| Sensing Materials | H2S Conc. (ppm) |
Response (Rg/Ra) |
LOD (ppm) |
Res. Time (s)/ Rec. Time (s) |
T (°C) |
Ref. |
|---|---|---|---|---|---|---|
| Pd-decorated CuO NWs | 100 | 1.962 | 1 | NA | 100 | [27] |
| Pd-decorated CuO nanorods | 100 | 31,243%(ΔR/Ra) × 100 | 20 | 670/80 | 300 | [48] |
| Pd (1 mol.%)-loaded CuO nanocrystals | 8 | 7.9 | 1 | NA | 250 | [49] |
| Pd-doped CuO nanoflowers | 50 | 123.4 | 0.1 | 15/3500 | 80 | [50] |
References
- Hu, Y.; Li, L.; Zhang, L.; Lv, Y. Dielectric barrier discharge plasma-assisted fabrication of g-C3N4-Mn3O4 composite for high-performance cataluminescence H2S gas sensor. Sens. Actuators B Chem. 2017, 239, 1177–1184.
- Li, H.; Liu, T.; Su, S.; Jin, M.; Fang, W.; Liu, L.; Wang, Y.; Hu, S.; Xiang, J. Effect of Ce modification on desulfurization per-formance of regenerated sorbent for high temperature H2S removal from coal gas. Fuel 2021, 293, 120463.
- Abdel Rahman, N.S.; Greish, Y.E.; Mahmoud, S.T.; Qamhieh, N.N.; El-Maghraby, H.F.; Zeze, D. Fabrication and characteri-zation of cellulose acetate-based nanofibers and nanofilms for H2S gas sensing application. Carbohydr. Polym. 2021, 258, 117643.
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331.
- Zheng, D.; Jiang, Z.; Shi, J.; Wang, Y.; Liu, Z. Experimental analysis of the effect of nitrogen gas on the H2S stripping process during the pigging operation of a long crude oil pipeline. Case Stud. Therm. Eng. 2020, 22, 100741.
- Hoa, T.T.N.; Le, D.T.T.; Van Toan, N.; Van Duy, N.; Hung, C.M.; Van Hieu, N.; Hoa, N.D. Highly selective H2S gas sensor based on WO3-coated SnO2 nanowires. Mater. Today Commun. 2021, 26, 102094.
- Ho, D.M.; Ho, B.Q.; Le, T.V. Evaluate of air pollution dispersion and propose planing scenerios to reduce air pollution for livestock activities in Tan Thanh district, Ba Ria—Vung Tau province. Sci. Technol. Dev. J. Sci. Earth Environ. 2019, 2, 26–37.
- Asad, M.; Sheikhi, M.H.; Pourfath, M.; Moradi, M. High sensitive and selective flexible H2S gas sensors based on Cu nanoparticle decorated SWCNTs. Sens. Actuators B Chem. 2015, 210, 1–8.
- Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918.
- Somacescu, S.; Stanoiu, A.; Dinu, I.V.; Calderon-Moreno, J.M.; Florea, O.G.; Florea, M.; Osiceanu, P.; Simion, C.E. CuWO4 with CuO and Cu(OH)2 native surface layers for H2S detection under in-field conditions. Materials 2021, 14, 465.
- Hsu, K.-C.; Fang, T.-H.; Hsiao, Y.-J.; Li, Z.-J. Rapid detection of low concentrations of H2S using CuO-doped ZnO nanofibers. J. Alloy. Compd. 2021, 852, 157014.
- Peng, F.; Sun, Y.; Lu, Y.; Yu, W.; Ge, M.; Shi, J.; Cong, R.; Hao, J.; Dai, N. Studies on Sensing Properties and Mechanism of CuO Nanoparticles to H2S Gas. Nanomaterials 2020, 10, 774.
- Ali, F.I.M.; Mahmoud, S.T.; Awwad, F.; Greish, Y.E.; Abu-Hani, A.F.S. Low power consumption and fast response H2S gas sensor based on a chitosan-CuO hybrid nanocomposite thin film. Carbohydr. Polym. 2020, 236, 116064.
- Hittini, W.; Abu-Hani, A.F.; Reddy, N.; Mahmoud, S.T. Cellulose-Copper Oxide hybrid nanocomposites membranes for H2S gas detection at low temperatures. Sci. Rep. 2020, 10, 1–9.
- Pravarthana, N.D.; Tyagi, A.; Jagadale, T.C.; Prellier, W.; Aswal, D.K. Highly sensitive and selective H2S gas sensor based on TiO2 thin films. Appl. Surf. Sci. 2021, 549, 149281.
- Mokoena, T.P.; Tshabalala, Z.P.; Hillie, K.T.; Swart, H.C.; Motaung, D.E. The blue luminescence of p-type NiO nanostructured material induced by defects: H2S gas sensing characteristics at a relatively low operating temperature. Appl. Surf. Sci. 2020, 525, 146002.
- Tang, Y.; Wu, W.; Wang, B.; Dai, X.; Xie, W.; Yang, Y.; Zhang, R.; Shi, X.; Zhu, H.; Luo, J.; et al. H2S gas sensing performance and mechanisms using CuO-Al2O3 composite films based on both surface acoustic wave and chemire-sistor techniques. Sens. Actuators B Chem. 2020, 325, 128742.
- Wu, Y.-Y.; Song, B.-Y.; Zhang, X.-F.; Deng, Z.-P.; Huo, L.-H.; Gao, S. Microtubular α-Fe2O3/Fe2(MoO4)3 heterostructure derived from absorbent cotton for enhanced ppb-level H2S gas-sensing performance. J. Alloy. Compd. 2021, 867, 158994.
- El-Shamy, A.G. New nano-composite based on carbon dots (CDots) decorated magnesium oxide (MgO) nano-particles () sensor for high H2S gas sensitivity performance. Sens. Actuators B Chem. 2021, 329, 129154.
- Yang, D.; Chen, G.; Fu, J.; Zhu, Y.; Dai, Z.; Wu, L.; Liu, J. The mitigation performance of ventilation on the accident consequences of H2S-containing natural gas release. Process Saf. Environ. Prot. 2021, 148, 1327–1336.
- Yang, S.; Sun, J.; Xu, L.; Zhou, Q.; Chen, X.; Zhu, S.; Dong, B.; Lu, G.; Song, H. functionalized three–dimensional macroporous WO3: A application of selective H2S gas sensor for exhaled breath biomarker detection. Sens. Actuators B Chem. 2020, 324, 128725.
- Song, B.-Y.; Zhang, M.; Teng, Y.; Zhang, X.-F.; Deng, Z.-P.; Huo, L.-H.; Gao, S. Highly selective ppb-level H2S sensor for spendable detection of exhaled biomarker and pork freshness at low temperature: Mesoporous SnO2 hierarchical architectures derived from waste scallion root. Sens. Actuators B Chem. 2020, 307, 127662.
- Pandey, S.K.; Kim, K.-H.; Tang, K.-T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99.
- Vuong, N.M.; Chinh, N.D.; Huy, B.T.; Lee, Y.-I. CuO-Decorated ZnO hierarchical nanostructures as efficient and established sensing materials for H2S gas sensors. Sci. Rep. 2016, 6, 26736.
- Ali, F.I.M.; Awwad, F.; Greish, Y.E.; Mahmoud, S.T. Hydrogen Sulfide (H2S) Gas Sensor: A Review. IEEE Sens. J. 2019, 19, 2394–2407.
- Peng, F.; Sun, Y.; Yu, W.; Lu, Y.; Hao, J.; Cong, R.; Shi, J.; Ge, M.; Dai, N. Gas sensing performance and mechanism of CuO(p)-WO3(n) composites to H2S gas. Nanomaterials 2020, 10, 1162.
- Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965.
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostruc-tures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141.
- Mirzaei, A.; Neri, G. Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: A review. Sens. Actuators B Chem. 2016, 237, 749–775.
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas sensing based on organic composite materials: Review of sensor types, progresses and challenges. Mater. Sci. Semicond. Process. 2021, 128, 105744.
- Ayesh, A.I.; Abu-Hani, A.F.; Mahmoud, S.T.; Haik, Y. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes. Sens. Actuators B Chem. 2016, 231, 593–600.
- Boepple, M.; Zhu, Z.; Hu, X.; Weimar, U.; Barsan, N. Impact of heterostructures on hydrogen sulfide sensing: Example of core-shell CuO/CuFe2O4 nanostructures. Sens. Actuators B Chem. 2020, 321, 128523.
- Nadargi, D.Y.; Tamboli, M.S.; Patil, S.S.; Dateer, R.B.; Mulla, I.S.; Choi, H.; Suryavanshi, S.S. Microwave-Epoxide-Assisted Hydrothermal Synthesis of the CuO/ZnO Heterojunction: A Highly Versatile Route to Develop H2S Gas Sensors. ACS Omega 2020, 5, 8587–8595.
- Paul, A.; Weinberger, C.; Tiemann, M.; Wagner, T. Copper Oxide/Silica Nanocomposites for Selective and Stable H2S Gas Detection. ACS Appl. Nano Mater. 2019, 2, 3335–3338.
- Balsamo, M.; Cimino, S.; de Falco, G.; Erto, A.; Lisi, L. ZnO-CuO supported on activated carbon for H2S removal at room temperature. Chem. Eng. J. 2016, 304, 399–407.
- Van Toan, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Le, D.T.T.; Viet, N.N.; Phuoc, P.H.; Van Hieu, N. Enhanced NH3 and H2 gas sensing with H2S gas interference using multilayer SnO2/Pt/WO3 nanofilms. J. Hazard. Mater. 2021, 412, 125181.
- Hu, Q.; Zhang, W.; Wang, X.; Wang, Q.; Huang, B.; Li, Y.; Hua, X.; Liu, G.; Li, B.; Zhou, J.; et al. Binder-free CuO nanoneedle arrays based tube-type sensor for H2S gas sensing. Sens. Actuators B Chem. 2021, 326, 128993.
- Huang, Z.; Wang, X.; Sun, F.; Fan, C.; Sun, Y.; Jia, F.; Yin, G.; Zhou, T.; Liu, B. Super response and selectivity to H2S at room temperature based on CuO nanomaterials prepared by seed-induced hydrothermal growth. Mater. Des. 2021, 201, 109507.
- Miao, J.; Chen, C.; Lin, J.Y. Humidity independent hydrogen sulfide sensing response achieved with monolayer film of CuO nanosheets. Sens. Actuators B Chem. 2020, 309, 127785.
- Dhakshinamoorthy, J.; Pullithadathil, B. New Insights Towards Electron Transport Mechanism of Highly Efficient p-Type CuO (111) Nanocuboids-Based H2S Gas Sensor. J. Phys. Chem. C 2016, 120, 4087–4096.
- Zhang, F.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J.; Qin, Y. CuO Nanosheets for Sensitive and Selective Determination of H2S with High Recovery Ability. J. Phys. Chem. C 2010, 114, 19214–19219.
- Li, X.; Wang, Y.; Lei, Y.; Gu, Z. Highly sensitive H2S sensor based on template-synthesized CuO nanowires. RSC Adv. 2012, 2, 2302–2307.
- Hosseini, Z.S.; Mortezaali, A.; Iraji, A.; Fardindoost, S. Sensitive and selective room temperature H2S gas sensor based on Au sensitized vertical ZnO nanorods with flower-like structures. J. Alloy. Compd. 2015, 628, 222–229.
- Zhou, Q.; Xu, L.; Umar, A.; Chen, W.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B Chem. 2018, 256, 656–664.
- Ngoc, T.M.; Van Duy, N.; Hung, C.M.; Hoa, N.D.; Nguyen, H.; Tonezzer, M.; Van Hieu, N. Self-heated Ag-decorated SnO2 nanowires with low power consumption used as a predictive virtual multisensor for H2S-selective sensing. Anal. Chim. Acta 2019, 1069, 108–116.
- Sarıca, N.; Alev, O.; Arslan, L.Ç.; Öztürk, Z.Z. Characterization and gas sensing performances of noble metals decorated CuO nanorods. Thin Solid Films 2019, 685, 321–328.
- Rydosz, A.; Maziarz, W.; Pisarkiewicz, T.; Wincza, K.; Gruszczyński, S. Nano-thin CuO films doped with Au and Pd for gas sensors applications. In Proceedings of the International Conference on Informatics, Electronics & Vision, Dhaka, Bangladesh, 17–18 May 2013; pp. 1–5.
- Kim, H.; Jin, C.; Park, S.; Kim, S.; Lee, C. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods. Sens. Actuators B Chem. 2012, 161, 594–599.
- Mikami, K.; Kido, Y.; Akaishi, Y.; Quitain, A.; Kida, T. Synthesis of Cu2O/CuO nanocrystals and their application to H2S sensing. Sensors 2019, 19, 211.
- Hu, X.; Zhu, Z.; Chen, C.; Wen, T.; Zhao, X.; Xie, L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sens. Actuators B Chem. 2017, 253, 809–817.
More
Information
Subjects:
Engineering, Chemical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
04 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




