
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rossella Tomaiuolo | + 2185 word(s) | 2185 | 2021-06-18 10:00:14 | | | |
| 2 | Camila Xu | + 364 word(s) | 2549 | 2021-06-22 11:17:23 | | |
Video Upload Options
The gender impact assessment (GIA) can be defined as an ex ante or ex post evaluation, analysis, or assessment of law, policy, or programme that helps to identify the likelihood of a decision having negative consequences for equality between women and men. GIA is aimed at improving the design and planning policy to prevent a negative effect on gender equality and improve gender equality through gender-oriented strategies.
1. Introduction
Infertility affects about 15–20% of couples worldwide [1]. Despite male-factor infertility being thought to play a role in 50% of infertile couples [2], currently, most scientific studies place the male factor as a secondary consideration compared to the female factor, in contrast with other pathologies (cardiovascular, degenerative, neurological, etc.) [3]. This difference is significant when considering the psychological distress generated by infertility; there is no doubt that infertility is a stressor for a couple, but it is experienced differently by males and females [4][5].
Since procreation involves sex (biological aspects) and gender (the social construction of femininity and masculinity, which includes sociocultural and psychological aspects) [6], optimizing the diagnostic and therapeutic journey of infertility and promoting gender equality is a mandatory gender-sensitive approach.
The introduction of a sex and gender determinant in clinical practice can contribute favourably to the management of prevention, diagnosis, and treatment strategies, making health services more effective and efficient [7], as these factors influence the physiological aspect and the pathological course of diseases affecting both men and women [8].
To date, gender models affect the general behaviours of women and men, and therefore the reproductive behaviours [9][10]: although both women and men are deeply affected by the infertility diagnosis, their psychological response is significantly influenced by gender [11][12] and negatively impacts the effectiveness of diagnostic-therapeutic interventions [13][14][15]. It is necessary to analyse the relevance of gender for and within the couple and to evaluate the state of knowledge for bringing equality into the mainstream of activities [16]. Understanding the impact of gender in the development and management of reproductive health and infertility can benefit the couple in terms of intervention and outcome and provide a deeper understanding for researchers and clinicians [17].
This paper aims to assess the impact of the gender dimension on the diagnostic-therapeutic journey of infertile couples and identify some crucial intervention points in order to address the gender balance. In particular, we adopted the gender impact assessment (GIA) method, validated for law and social issues, and applied it to a public health system [18].
2. Materials and Methods
A non-systematic review was done through a search on the following databases: MEDLINE, EMBASE, Global Health, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register), Health Technology Assessment Database, Web of Science, and research registers (such aswww.cliniclatrials.gov(accessed on 1 March 2021); we used the medical subject heading (MeSH) terms “Gender Identity” ( MeSH Unique ID: D005783) or “Gender Role” in combination with “Infertility” (MeSH Unique ID: D007246), “Infertility, Male” (MeSH Unique ID: D007248), “Infertility, Female”
Titles and/or abstracts of studies retrieved using the search strategy, and those from additional sources, were screened independently by two review authors (A.S.L. and R.T.) to identify studies that potentially meet the aims of this nonsystematic review. These potentially eligible articles’ full text was retrieved and independently assessed for eligibility by the other two review team members (G.G. and G.B.). Any disagreement between them over the eligibility of particular articles was resolved through discussion with a third (external) collaborator. Two authors (A.R.C. and P.G.) independently extracted data from articles about study features and included populations, type of intervention (duration of therapy and drug posology), and outcomes.
This method is a stepwise approach that, through the identification of relevant gender issues, picks up gendered effects and simulates gender equality outcomes. The basis for identifying gender relevance is to disaggregate the data by sex, subsequently, carry out the full-fledged gender impact assessment, and finally address the gender balance with suggestions for reducing gender inequalities and promoting gender equality. Pretest of checking gender relevance. Addressing the gender balance, giving suggestions for reducing gender inequalities and improving gender equality.
Every article included in the review was carefully read, and qualitative data were identified and extracted. Key themes have been identified and used to create a narrative discussion due to the difficulty in obtaining a quantifiable parameter. The parameters considered as relevant were the following: Characteristics of the subjects: age/date of birth, nationality, educational qualification, profession, religion, and relationship with ART. Characteristics of the families of origin: age of parents; profession; living distances; welfare needs; years of marriage and procreative research; sequential reconstruction of the family story accompanied by age, marital status, and presence of children; the possible presence of cases of abortion or sterility in the family; and any genetically transmitted diseases or infections. ; significant experiences faced together; the story of the personal process of procreative waiting and health; causes of infertility and any surgical intervention (e.g., varicocele, endometriosis, etc.); and previous ART.Psychological interview: biopsychosocial data collection, its usefulness, and other contacts with psychologists in the past.
3. Discussion
Gender bias in health involves biological sex differences and gender differences in the way women and men behave and how they are treated [17]. However, we argue that the gender impact assessment can become a force for good in moving health practices towards gender equity by revealing and challenging gender bias. The gender impact assessment (GIA) can be defined as an ex-ante or ex-post evaluation, analysis, or assessment of law, policy, or programme that helps to identify the likelihood of a decision having negative consequences for equality between women and men [18]. GIA is aimed at improving the design and planning policy to prevent a negative effect on gender equality and improve gender equality through gender-oriented strategies [19].
To date, the GIA tool has been recently assessed for health practice [20]. In particular, a large cohort of healthcare employees participating in the Italian vaccination campaign against SARS-CoV-2 has been investigated to assess the impact of sex and gender on vaccination coverage using the GIA approach. However, couple infertility is perfect for GIA analysis due to the implication of both sex and gender factors. Since infertility always harms both partners, it can be the first healthcare issue in which a systematic gender impact assessment could be efficiently performed and subsequently used in other health settings.
The first step of GIA applied to the journey of couple infertility showed that gender is a relevant determinant. Although infertility affects both men and women equally, the in-depth analysis of the infertile couples’ diagnostic journey (Figure 2) shows that the female factor evaluation is the most consistent driver for the infertility workup.
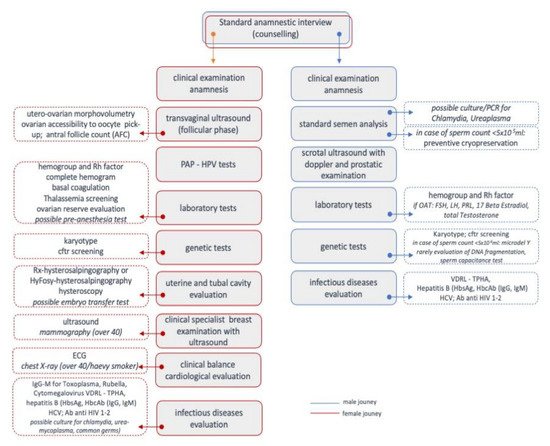
The full-fledged gender impact assessment (second step) focused on explaining the gender dynamics and identifying and evaluating gender impacts. Owing to social constructs, current gender dynamics mean that female factors are more likely to seek medical attention. In contrast, male factors are not systematically analysed and overlooked in the specific pathological conditions related to infertility [21].
The harmful impacts of gender bias were investigated through genetic testing and ART. Currently, male infertility’s genetic diagnostic workup, restricted to the karyotype, AZF region, and CFTR gene, is considered ineffective [22]. The number of genetic variants related to infertility is constantly increasing, providing a decrease in the current percentage of idiopathic male infertility [22]. Furthermore, numerous data suggest that genetic abnormalities involved in male infertility can also affect morbidity and life expectancy, suggesting a link between male infertility and oncological, cardiovascular, metabolic, and autoimmune diseases [2].
The ART, which has rapidly evolved during the last decades, led to overcoming the limitations due to male factor infertility [23], and in particular, the development of intracytoplasmic sperm injection (ICSI) can currently be considered a significant step forward Furthermore, ICSI has no clear advantages in patients with normal semen parameters and should be offered only in cases of severe male factor [24]. Indeed, ICSI is not associated with a significantly higher fertilization rate, clinical pregnancy rate, implantation rate, and live birth rate than standard IVF in couples without male factor infertility. Only recently, growing evidence suggests that sperm carries pivotal factors acquired intrinsically during spermatogenesis or extrinsically during storage and ejaculation in the male reproductive tract.
On this basis, in a future scenario, the male partner should be carefully managed by taking into account not only the sperm concentration, motility, and morphology, but also genetic tests and testicular histology in order to offer a tailored treatment and targeted use of ART [25].
Many couples state that infertility is the most stressful problem in their lives and that fertility treatments disrupt individuals’ and couples’ lives [6][26][27][28][29]. Specifically, the large body of literature focused on different variables (behavioural, relational, social, emotional, and cognitive) across treatment stages (before, during, and after treatment) involved in infertility Moreover, several studies show that psychological stress among men during infertility treatment is not associated with testicular function [30]. Secondly, it has also been highlighted that men report higher social isolation than women and are not likely to refer to psychosocial services [31].
What can be learned from many of the studies focusing on cognitive, emotional, behavioural, relational, and social issues highlights both men and women as well as the relevance of offering specific psychological counselling to both because they are strongly connected to each other. Individuals who perceive their partner to be available and responsive experience lower infertility stress than individuals who perceive their partner as avoidant and nonresponsive [32][33]. A partner’s use of active-avoidance coping is related to higher marital distress for men and women [34]; a partner’s use of meaning-based coping is associated with lower marital distress in men, reinforcing the dyadic nature of data when approaching infertility issues. Based on these data, psychological interventions detecting and treating individual and dyadic psychological needs of couples involved in fertility journeys are strongly recommended.
Taking all these data together, we can summarize that the GIA of the diagnostic-therapeutic journey of the infertile couple has brought out some key points. All data are grouped together in table 1, where the state of the art according to our review is presented, and the strategies to reduce the gender differences impact on ART are proposed.
Table 1. The problems affecting gender impact in ART are presented as “as is”. Strategies to reduce gender differences impact are presented as “to be”. Results are strictly linked to gynaecological and genetic assessment strategies in ART.
| AS IS (Problems) | TO BE (Solutions) |
|---|---|
| The erroneous concept that full investigation for infertile men is not needed | Male fertility experts should always be involved in the diagnostic process of the infertile couple |
| Male infertility is usually defined only based on semen analysis | Assessment should embrace:
|
| Semen reporting is still performed in many laboratories that do not have adequate preparation | Semen should be evaluated according to the World Health Organization (WHO) manual and preferably performed in laboratories that have expertise in reproductive medicine |
A common malpractice is:
|
Solutions are:
|
| Multiple cycles of IVF/ICSI can last for years and the male figure must not be neglected during the months of treatment, limiting itself to the sole observation of the seminal fluid values | In addition, given the strong association between infertility, cryptorchidism, testicular hypotrophy, and microlithiasis with testicular cancer, recurring scrotal ultrasonography is a great opportunity to identify suspected testis masses and nodules |
Genetic variables are studied:
|
Solutions are:
|
First, there is a strong tendency towards medicalization rather than preserving healthy reproduction. ART represents the apical moment of the reproductive medicalization process, bypassing the couple’s infertility rather than resolving the causes. ART is increasingly used, neglecting that in many cases, infertility can be prevented with effective awareness campaigns (age, sexually transmitted diseases, lifestyle, and environmental pollution) or resolved with in-depth targeted therapeutic, diagnostic interventions [35]. Where the indications allow it, the therapeutic choices should follow the principle of graduality and should be differentiated into subsequent steps [22].
Therefore, both partners must undergo thorough investigations to rule out potentially reversible causes of infertility and improve the ability to procreate naturally. Furthermore, the negative impact of an unbalanced diagnostic process is reflected in the lack of diagnosis for the underlying causes of male infertility. Improving the detection rate of genetic tests also becomes essential because male infertility is associated with poorer overall health, increased cancer risk, and decreased life expectancy. Therefore, general practitioners have a pivotal role in educating patients about modifiable factors, maximizing the fertility potential, and improving the male patient’s
The third consideration that emerged is the male partner’s psychological distress, mainly due to the different involvement in the whole diagnostic-therapeutic process [8]. Infertility should be addressed using a gender-specific-related approach. Indeed, the gender-specific approach takes into account the recent criteria of intersectionality [36]: it highlights how various biological, social, and cultural categorizations (for example, gender, ethnicity, social class, disability, sexual orientation, religion, age, nationality, species, and other interconnected axes of identity) interact at multiple levels, often simultaneously, being able to create factors of discrimination and inequalities [37][38]. Through an anamnesis conducted with the awareness of intersectionality, the differences and traits of a person are recognized as inextricably linked to all the other elements, with the advantage of being able to understand better that person’s identity and more or less manifest needs and being able to both cure it
For example, it has been shown that significantly more males are born after blastocyst transfer in IVF cycles [39]. Although further studies are needed to detect real differences and aetiology, this issue makes the gender impact debate intriguing. This effort is made by ESHRE and finds its main key points in milder stimulation protocols, single-embryo transfers, and cost-effectiveness of infertility diagnosis and treatment [40]. Since LCIVF may become the future of ART in developing countries, a strong effort should, in our opinion, be made ex-ante in assessing gender impact and preventing gender differences.
4. Conclusions
The integration of scientific knowledge with gender-specific determinants has brought out some crucial points of intervention (Figure 3): the need to implement information interventions on preventable causes of infertility; customize diagnostic pathways to identify specific causes of male infertility to cure it rather than bypass its effects; analyse the couple simultaneously, and use ART according to the criterion of graduality.
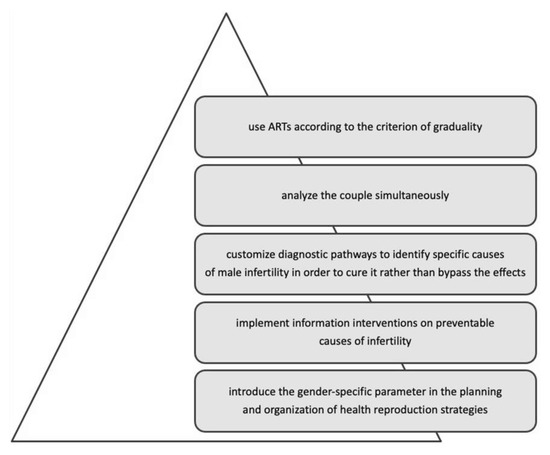
In conclusion, including the gender dimension throughout the diagnostic-therapeutic journey of infertile couples helps eliminate gender bias, indicating how to design equitable access to infertility diagnosis and treatment.
References
- Pathak, U.I.; Gabrielsen, J.S.; Lipshultz, L.I. Cutting-Edge Evaluation of Male Infertility. Urol. Clin. N. Am. 2020, 47, 129–138.
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814.
- Sylvest, R.; Fürbringer, J.K.; Schmidt, L.; Pinborg, A. Infertile men’s needs and assessment of fertility care. Upsala J. Med. Sci. 2016, 121, 276–282.
- Peterson, B.D.; Newton, C.R.; Rosen, K.H.; Skaggs, G.E. Gender differences in how men and women who are referred for IVF cope with infertility stress. Hum. Reprod. 2006, 21, 2443–2449.
- Ying, L.Y.; Wu, L.H.; Loke, A.Y. Gender differences in experiences with and adjustments to infertility: A literature review. Int. J. Nurs. Stud. 2015, 52, 1640–1652.
- Greil, A.L.; Shreffler, K.M.; Schmidt, L.; McQuillan, J. Variation in distress among women with infertility: Evidence from a population-based sample. Hum. Reprod. 2011, 26, 2101–2112.
- Day, S.; Mason, R.; Lagosky, S.; Rochon, P.A. Integrating and evaluating sex and gender in health research. Health Res. Policy Syst. 2016, 14, 1–5.
- Baggio, G.; Corsini, A.; Floreani, A.; Giannini, S.; Zagonel, V. Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 2013, 51, 713–727.
- Pinnelli, A.; Di Cesare, M. Human fertility: Sociodemographic aspects. Contraception 2005, 72, 303–307.
- Popay, J. Whose theory is it anyway? J. Epidemiol. Community Health 2006, 60, 571–572.
- Nachtigall, R.D.; Becker, G.; Wozny, M. The effects of gender-specific diagnosis on men’s and women’s response to infertility. Fertil. Steril. 1992, 57, 113–121.
- Casu, G.; Zaia, V.; Fernandes Martins, M.; Parente Barbosa, C.; Gremigni, P. A dyadic mediation study on social support, coping, and stress among couples starting fertility treatment. J. Fam. Psychol. 2019, 33, 315–326.
- Halcomb, L. Men and infertility: Insights from the sociology of gender. Sociol. Compass 2018, 12, e12624.
- Dudgeon, M.R.; Inhorn, M.C. Men’s influences on women’s reproductive health: Medical anthropological perspectives. Soc. Sci. Med. 2004, 59, 1379–1395.
- Fledderjohann, J.; Barnes, L.W. Reimagining infertility: A critical examination of fertility norms, geopolitics and survey bias. Health Policy Plan. 2018, 33, 34–40.
- Bíziková, L.; Sedová, T.; Szapuová, M. Why Gendered Science Matters. How to Include Gender Dimension into Research Projects. 2007. Available online: (accessed on 2 April 2021).
- Ovseiko, P.V.; Greenhalgh, T.; Adam, P.; Grant, J.; Hinrichs-Krapels, S.; Graham, K.E.; Valentine, P.A.; Sued, O.; Boukhris, O.F.; Al Olaqi, N.M.; et al. A global call for action to include gender in research impact assessment. Health Res. Policy Syst. 2016, 14, 1–12.
- Klinge, I. Bringing gender expertise to biomedical and health-related research. Gend. Med. 2007, 4, S59–S63.
- European Commission. Toolkit for Gender in Research: Checklist for Gender in Research. European Commission. 2011. Available online: (accessed on 2 April 2021).
- Di Resta, C.; Ferrari, D.; Viganò, M.; Moro, M.; Sabetta, E.; Minerva, M.; Ambrosio, A.; Locatelli, M.; Tomaiuolo, R. The Gender Impact Assessment among Healthcare Workers in the SARS-CoV-2 Vaccination—An Analysis of Serological Response and Side Effects. Vaccines 2021, 9, 522.
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: A committee opinion. Fertil. Steril. 2015, 103, e18–e25.
- Cariati, F.; D’Argenio, V.; Tomaiuolo, R. The evolving role of genetic tests in reproductive medicine. J. Transl. Med. 2019, 17, 267.
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Research 2019, 8, 670.
- Geng, T.; Cheng, L.; Ge, C.; Zhang, Y. The effect of ICSI in infertility couples with non-male factor: A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2020, 37, 2929–2945.
- Ferlin, A.; Foresta, C. Infertility: Practical Clinical Issues for Routine Investigation of the Male Partner. J. Clin. Med. 2020, 9, 1644.
- Gameiro, S.; Boivin, J.; Dancet, E.; De Klerk, C.; Emery, M.; Lewis-Jones, C.; Thorn, P.; Broeck, U.V.D.; Venetis, C.; Verhaak, C.; et al. ESHRE guideline: Routine psychosocial care in infertility and medically assisted reproduction—A guide for fertility staff. Hum. Reprod. 2015, 30, 2476–2485.
- Donarelli, Z.; Lo Coco, G.; Gullo, S.; Salerno, L.; Marino, A.; Sammartano, F.; Allegra, A. The Fertility Quality of Life Questionnaire (FertiQoL) Relational subscale: Psychometric properties and discriminant validity across gender. Hum. Reprod. 2016, 31, 2061–2071.
- Donarelli, Z.; Salerno, L.; Lo Coco, G.; Allegra, A.; Marino, A.; Kivlighan, D.M. From telescope to binoculars. Dyadic outcome resulting from psychological counselling for infertile couples undergoing ART. J. Reprod. Infant Psychol. 2019, 37, 13–25.
- Matthiesen, S.M.; Frederiksen, Y.; Ingerslev, H.J.; Zachariae, R. Stress, distress and outcome of assisted reproductive technology (ART): A meta-analysis. Hum. Reprod. 2011, 26, 2763–2776.
- Bräuner, E.V.; Nordkap, L.; Priskorn, L.; Hansen, Å.M.; Bang, A.K.; Holmboe, S.A.; Schmidt, L.; Jensen, T.K.; Jørgensen, N. Psychological stress, stressful life events, male factor infertility, and testicular function: A cross-sectional study. Fertil. Steril. 2020, 113, 865–875.
- Schmidt, L.; Holstein, B.E.; Boivin, J.; Sångren, H.; Tjørnhøj-Thomsen, T.; Blaabjerg, J.; Hald, F.; Andersen, A.N.; Rasmussen, P.E. Patients’ attitudes to medical and psychosocial aspects of care in fertility clinics: Findings from the Copenhagen Multi-centre Psychosocial Infertility (COMPI) Research Programme. Hum. Reprod. 2003, 18, 628–637.
- Van den Broeck, U.; D’Hooghe, T.; Enzlin, P.; Demyttenaere, K. Predictors of psychological distress in patients starting IVF treatment: Infertility-specific versus general psychological characteristics. Hum. Reprod. 2010, 25, 1471–1480.
- Donarelli, Z.; Lo Coco, G.; Gullo, S.; Marino, A.; Volpes, A.; Allegra, A. Are attachment dimensions associated with infertility-related stress in couples undergoing their first IVF treatment? A study on the individual and cross-partner effect. Hum. Reprod. 2012, 27, 3215–3225.
- Peterson, B.D.; Sejbaek, C.S.; Pirritano, M.; Schmidt, L. Are severe depressive symptoms associated with infertility-related distress in individuals and their partners? Hum. Reprod. 2014, 29, 76–82.
- D’Argenio, V.; Cariati, F.; Tomaiuolo, R. One4Two®: An Integrated Molecular Approach to Optimize Infertile Couples’ Journey. Genes 2021, 12, 60.
- Crenshaw, K. Demarginalizing the Intersection of Race and Sex: A Black Feminist Critique of Antidiscrimination Doctrine. Feminist Theory and Antiracist Politics. Univ. Chic. Leg. Forum. 1989, 140, 139–167.
- Damaskos, P.; Amaya, B.; Gordon, R.; Walters, C.B. Intersectionality and the LGBT Cancer Patient. Semin. Oncol. Nurs. 2018, 34, 30–36.
- Mena, E.; Bolte, G. Advance gender Study Group. Intersectionality-based quantitative health research and sex/gender sensitivity: A scoping review. Int. J. Equity Health 2019, 18, 1–11.
- Hentemann, M.A.; Briskemyr, S.; Bertheussen, K. Blastocyst transfer and gender: IVF versus ICSI. J. Assist. Reprod. Genet. 2009, 26, 433–436.
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426.




