Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuen-Ei Chen | + 2850 word(s) | 2850 | 2021-06-17 10:26:34 | | | |
| 2 | Rita Xu | -8 word(s) | 2842 | 2021-06-18 09:43:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, S. Broiler Breeder Hens. Encyclopedia. Available online: https://encyclopedia.pub/entry/11002 (accessed on 08 February 2026).
Chen S. Broiler Breeder Hens. Encyclopedia. Available at: https://encyclopedia.pub/entry/11002. Accessed February 08, 2026.
Chen, Shuen-Ei. "Broiler Breeder Hens" Encyclopedia, https://encyclopedia.pub/entry/11002 (accessed February 08, 2026).
Chen, S. (2021, June 18). Broiler Breeder Hens. In Encyclopedia. https://encyclopedia.pub/entry/11002
Chen, Shuen-Ei. "Broiler Breeder Hens." Encyclopedia. Web. 18 June, 2021.
Copy Citation
Past immunological studies in broilers focused on juveniles within the rapid pre-slaughter growth period and may not reflect adult immune responses, particularly in breeders managed with chronic feed restriction (R).
broiler breeder hens
25-hydroxycholecalciferol
innate immunity
glucolipotoxicity
1. Introduction
Modern broilers can reach a market weight of ~2 kg within 35 days, half of the time needed by their ancestors 50 years ago. Genetic selection expedited growth, but also resulted in reproductive inefficacy, and increased susceptibility to sudden death syndrome, fatty liver syndrome, and obesity-related morbidities such as cardiomyopathy [1][2][3][4]. Feed restriction is typically used to achieve target bodyweight gain in broiler breeders and improve reproductive performance and livability by limiting obesity and related dysfunctions [1][2][3][4].
Several studies found that immunological response was also a trade-off for rapid growth selection [5][6][7][8]. Genetic selection for improved growth performance has resulted in a decline of adaptive immune responses co-incident with an increase in cellular immunity and inflammatory responses [8]. To date, most of the immunological studies in growth selected lines of chickens were performed in juvenile birds during the 6 weeks prior to slaughter when massive lean tissue gain occurs. However, the effect of chronic feed restriction on the ability to develop optimal immunity in response to vaccination or against pathogen infection in breeder chickens is sparsely studied.
Increased fat mass in obese patients can induce metabolic dysregulation. It has also been recognized that obesity development is tightly associated with inflammatory status, particularly in the adipose tissue [9][10]. The abnormal provocation of factors of innate and adaptive immune system by inflammation acts as a strong inducer in the development of type 2 diabetes mellitus (T2DM) [9][11]. Our previous studies showed many metabolic commonalties existing between obese (T2DM) and broiler breeder hens consuming feed to appetite. Indeed, we have shown that prolonged Ad-feed intake by broiler breeder hens causes rapid fat deposition and produces lipotoxicity and an inflammatory response arising from lipid dysregulation and associated changes in gene expression and signaling that results in impaired functions in ovarian follicles, circulating leukocytes, heart, and pancreatic β-cells [3][4][12][13][14][15].
Innate immunity functions as the frontline defense against pathogen invasions; moreover, it also directs cellular and humoral responses to eliminate pathogens. Neutrophils and macrophages are key cells in the innate immune response against invading pathogens. Both types of cells function to clear pathogens through their phagocytic capacity and generation of oxidants that kill engulfed microorganisms [12]. The clearance of pathogens and apoptotic cells by phagocytic cells plays an important role in resolving inflammatory responses, and impairments of these processes often result in a chronic inflammatory state [16][17].
In a variety of models, vitamin D and its receptor signaling play an anti-obesity and –inflammatory role in the development of T2DM and cardiac pathogenesis [18][19]. Vitamin D was shown to be a pivotal component of the monocyte/macrophage response to infection [20][21], inducing their antimicrobial activity. Vitamin D3 (VD3) supplementation not only reversed VD3 deficiency–induced inflammatory responses but also alleviated immunological inflammation caused by LPS (lipopolysaccharide) in table-egg type laying hens [22][23]. We found that dietary 25-hydroxycholecalciferol (25-OH-D3) supplementation improved cardiac health and rescued the livability in the breeder hens partially by ameliorating systemic and cardiac inflammation and fibrotic progression [24][25][26][27].
2. Body Weight, Feed Intake, Plasma Glucose, Triglyceride, NEFA, Insulin, and IL-1β Levels
Regardless of 25-OH-D3 supplementation, Ad-feed intake showed an initial burst of feed consumption in the first week from the prescribed 157 to 196 g/day/hen. Feed intake then declined gradually to reach a nadir at 62 weeks (132 g/day/hen), and thereafter increased slowly to 153 g/day/hen at 68 weeks. The BW of Ad-hens increased sharply to reach 4.6 kg/hen at age of 60 weeks, declined slightly to 63–64 weeks, and subsequently, increased to reach 4.6–4.7 kg/hen at 68 weeks. This pattern of feed intake and BW gain was in marked contrast to a slow increase of BW from ~3.6 kg at 51 weeks to ~3.95 kg at 68 weeks with the breeder prescribed feed allotments.
Ad-feed intake provoked obesity-associated metabolic dysregulations including increased fasting plasma glucose levels and 30 min re-fed plasma glucose concentrations at 65 weeks. Increases in circulating insulin, TG, and NEFA concentrations were observed in Ad-hens at both 58 and 65 weeks (p < 0.05, Figure 1A–D). Dietary 25-OH-D3 supplementation suppressed plasma glucose, insulin and NEFA levels of Ad-hens at 65 weeks but did not affect concentrations in R-hens. Ad-feed intake also provoked chronic systemic inflammation as shown by increased plasma IL-1β concentrations at 58 and 65 weeks. Supplemental 25-OH-D3 significantly suppressed plasma IL-1β levels in both R- and Ad-hens at 65 weeks (p < 0.05, Figure 1E). A significant interaction between Ad and 25-OH-D3 treatments was found for plasma glucose, NEFA, and IL-1β level at 65 weeks (p < 0.05, Figure 1A–D) as 25-OH-D3 decreased values more in Ad-hens than in R-hens.
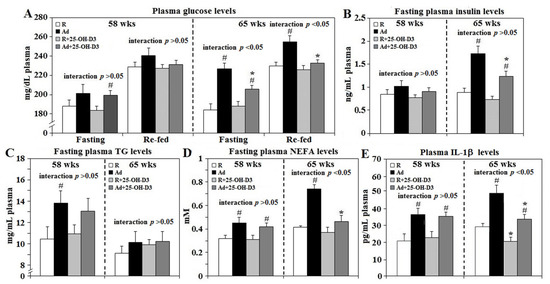
Figure 1. Plasma glucose, insulin, TG, NEFA, and IL-1β concentrations of broiler breeder hens provided with restricted (R) or ad libitum (Ad) feed intake. At age of 58 and 65 weeks (wks), 6 hens from each group were randomly selected for blood collection for plasma glucose, insulin, triacylglycerol (TG), non-esterified fatty acid (NEFA), and interleukin-1β (IL-1β) analysis (A–E, respectively, n = 6). #; significant difference by Ad-feed intake (vs. corresponding R hens, p < 0.05), *; significant difference by 25-OH-D3 (vs. R- or Ad-hens, p < 0.05). 25-OH-D3: 25-hydroxycholecalciferol.
3. IL-1β Secretion, Phagocytosis, and Respiratory Burst of Fresh Leukocytes
In contrast to R-hens, freshly isolated heterophils at 67–68 weeks and monocytes at both 59–60 and 67–68 weeks from Ad-hens had lower IL-1β secretion (p < 0.05, Figure 2A). Supplemental 25-OH-D3 suppressed heterophil IL-1β secretion in both R- and Ad-hens at 59–60 weeks and in Ad- and R-hen monocytes at 59–60 and 67–68 weeks, respectively, but increased heterophil IL-1β secretion in Ad-hens at 67–68 weeks. Ad-hen monocytes had a lower phagocytic activity at 67–68 weeks and supplemental 25-OH-D3 increased the phagocytosis of Ad-hen heterophils and monocytes at 67–68 weeks and at 59–60 weeks, respectively (p < 0.05, Figure 2B). Ad-feed intake also significantly suppressed respiratory burst response in both types of leukocytes at 59–60 and 67–68 weeks (p < 0.05), which 25-OH-D3 did not alter in Ad-hens. This same variable was significantly decreased in R-hen heterophils and monocytes at 59–60 and/or 67–68 weeks (p < 0.05, Figure 2C) with 25-OH-D3 supplementation. A significant interaction between Ad×25-OH-D3 treatments was observed in IL-1β secretion and phagocytosis of both cell types at 59–60 and/or 67–68 weeks, and in the respiratory burst response of heterophils at 59–60 and 67–68 weeks (p < 0.05, Figure 2A–C).
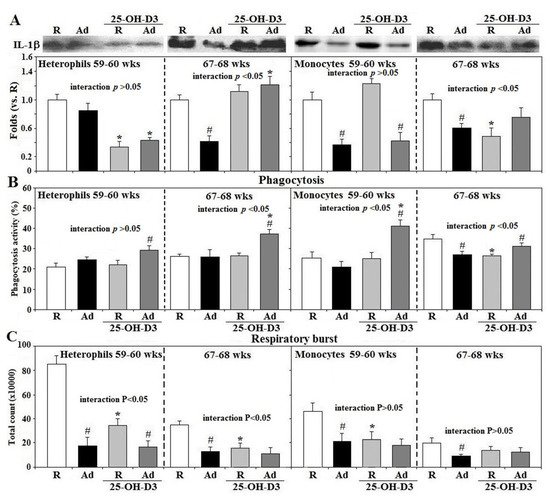
Figure 2. Effects of dietary supplementation of 25-OH-D3 on IL-1β secretion, phagocytosis, and the respiratory burst of leukocytes in broiler breeder hens provided with restricted (R) or ad libitum (Ad) feed intake. Peripheral leukocytes (2 × 106 cells) isolated from hens at age 59–60 and 67–68 weeks (wks) were cultured for 3 h and medium were then collected for interleukin-1β (IL-1β) analysis (A) by Western blot method based on equivalent amounts of protein (n = 6). Freshly prepared cells were used for phagocytosis analysis (B) and respiratory burst analysis (C) (n = 6). Chemiluminescence results of respiratory burst analysis were expressed as integrated counts over 45 min. Results of Western blots were expressed as rations relative to R-hens. *; significant difference by 25-OH-D3, p < 0.05, #; significant difference by Ad-feed intake. 25-OH-D3; 25-hydroxycholecalciferol.
4. Chemotaxis and Bacterial Killing of Fresh Leukocytes
Ad-feed intake exerted no significant effects on chemotaxis and bactericidal activity in either type of leukocytes. However, supplemental 25-OH-D3 increased monocyte chemotaxis in both Ad- and R-hens, as well as bactericidal activity in Ad-hens (p < 0.05, Figure 3A,B). Viability of both types of leukocytes was decreased in Ad-hens compared to those of R-hens. Early apoptosis accounted for most of the heterophil death in both R- and Ad-hens, whereas late apoptosis contributed most of the death in monocytes (p < 0.05, Figure 3C). Supplemental 25-OH-D3 had no effects on the viability of either cell type.
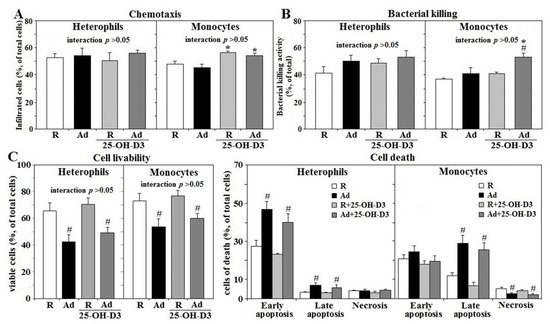
Figure 3. Effects of dietary supplementation of 25-OH-D3 on chemotaxis, bacterial killing and cell livability of leukocytes in broiler breeder hens under restricted (R) or ad libitum (Ad) feed intake. Freshly prepared cells (2 × 105) from hens at age of 66 weeks were used for chemotaxis analysis through trans-well method (A), incubated with Salmonella Typhimurium (ST) at a 1:2 ratio for 45 min for bacterial killing analysis (B), or suspended in RPMI for cell livability analysis (C) (n = 6). Cell viability was defined as the percentage of total cells present that were viable in a cell death analysis with FITC-Annexin-V/propidium iodide (PI) staining and cytometry sorting. Results of bacterial killing were expressed as ratios relative to R-hens. *; significant difference by 25-OH-D3, p < 0.05, #; significant difference by Ad-feed intake. 25-OH-D3; 25-hydroxycholecalciferol.
5. VDR Protein Amounts and NFκB Activation of Fresh Leukocytes
Ad-feed intake exerted no significant effects on VDR expression and p65 activation in both types of cells and supplemental 25-OH-D3 upregulated heterophil and monocyte VDR expression in both R- and Ad-hens, but suppressed heterophil p65 translocation (p < 0.05, Figure 4A–C). The results confirmed the inhibitory action of supplemental 25-OH-D3 on NFκB signaling in the animal model.
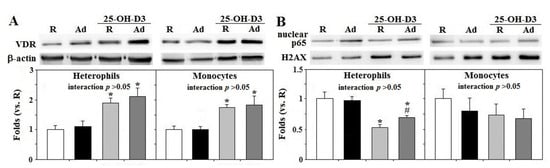
Figure 4. Effects of dietary supplementation of 25-OH-D3 on VDR protein amounts and p65 activation of leukocytes in broiler breeder hens provided with restricted (R) or ad libitum (Ad) feed intake. Freshly prepared cells from hens at the age of 66 weeks were used for total protein extraction for vitamin D receptor (VDR) expression (A) and nuclear extracts were used for p65 (a subunit of nuclear factor kappa B, NFκB) translocation (B) through Western blot method. Results were normalized to β-actin or H2AX and expressed as ratios relative to R-hens (n = 6). *; significant difference by 25-OH-D3, p < 0.05, #; significant difference by Ad-feed intake. 25-OH-D3; 25-hydroxycholecalciferol.
6. Effects of 25-OH-D3 on IL-1β Secretion of Leukocytes
In order to delineate the effects of 25-OH-D3 on innate immune functions of R- or Ad-fed hens, isolated leukocytes from R-hens were used for overnight culture studies. Treatment with 25-OH-D3 increased IL-1β secretion in heterophils in a dose-dependent manner, but decreased IL-1β secretion in monocytes (p < 0.05, Figure 5A). 25-OH-D3 differentially promoted phagocytosis and respiratory burst response in both types of leukocytes (p < 0.05, Figure 5B,C). Based on these results, the dose for 25-OH-D3 was optimized at 50 nM for the following studies.
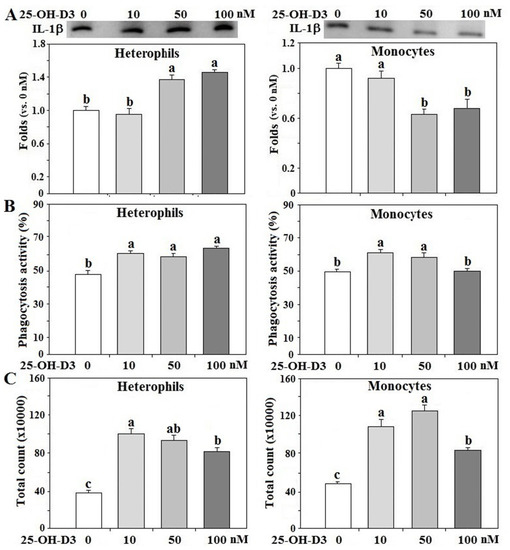
Figure 5. Effects of 25-OH-D3 on leukocyte functions. Peripheral heterophils and monocytes (2 × 106 cells) isolated from R-hens at age 61–65 weeks were treated with various levels of 25-hydroxycholecalciferol (25-OH-D3) overnight (16 h). Media were collected for interleukin-1β (IL-1β) analysis by Western blot method based on protein equivalency (A); cells were collected for phagocytosis (B) and respiratory burst analysis (C) (n = 4). Chemiluminescence results of respiratory burst analysis were expressed as integrated counts over 45 min. Results of Western blots were expressed as ratios relative to control (0 nM of 25-OH-D3). Means with different letters differ significantly (p < 0.05).
7. Effects of Glucose and Fatty Acid on Leukocyte Functions
Treatment with glucose suppressed IL-1β secretion, phagocytosis, and respiratory burst response in both types of leukocytes in a dose-dependent manner (p < 0.05, Figure 6A–C). Since the glucose treatment at 300 mg/dL completely blocked the respiratory burst response in heterophils, and glucose at 100 mg/dL failed to affect IL-1β secretion, the dose for glucose effects in the mechanistic studies was optimized at 200 mg/dL. A dose-dependent suppression of IL-1β secretion and phagocytic activity by PA treatment was also observed in both cell types (p < 0.05, Figure 6D,E). Interestingly, PA treatment suppressed heterophil respiratory burst in a dose-dependent fashion, but increased the response in monocytes (p < 0.05, Figure 6F). The dose for PA effects on following studies was optimized at 1.5 mM.
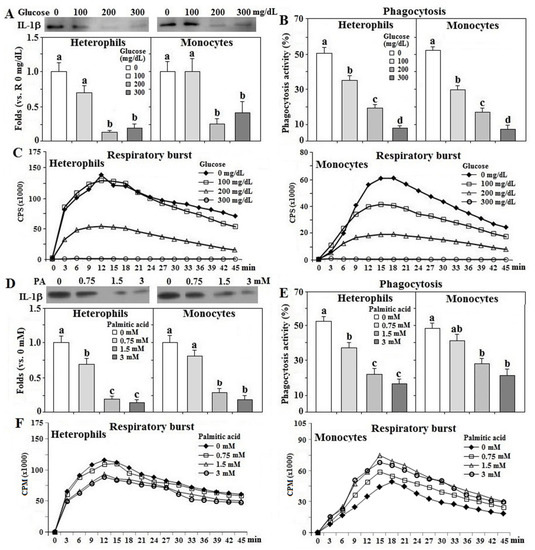
Figure 6. Effects of glucose and palmitic acid on leukocyte functions. Peripheral heterophils and monocytes (2 × 106 cells) isolated from R-hens at age 61–65 weeks were treated with various levels of glucose or palmitic acid (PA) overnight (16 h). Media were collected for interleukin-1β (IL-1β) analysis by Western blot method based on equivalent amounts of protein (A,D) and collected cells were used for phagocytosis analysis (B,E) and respiratory burst analysis (C,F) (n = 4). Chemiluminescence results of respiratory burst analysis were expressed as count per second (CPM) over 45 min. Results of Western blots were expressed as rations relative to control (no glucose or PA supplementation). Means with different letters differ significantly (p < 0.05).
8. Effects of 25-OH-D3 on Leukocyte Functions during Glucolipotoxicity
Treatment of glucose or PA alone impaired IL-1β secretion in both heterophils and monocytes (p < 0.05) and combination of glucose and PA did not exacerbate the impairment (Figure 7A). It was shown that 25-OH-D3 had no effects on IL-1β secretion impaired by glucose, PA alone, or by their combination in either cell type. In the presence of 25-OH-D3, but not its absence, PA treatment further reduced IL-1β secretion caused by monocyte treatment with glucose (p < 0.05, Figure 7A). Combination of glucose and PA exacerbated phagocytic activity impaired by glucose or PA alone in both types of leukocytes and treatment of 25-OH-D3 rescued phagocytosis impaired by glucose and PA combination (p < 0.05, Figure 7B). In heterophils, glucose or PA alone impaired respiratory burst, while the addition of PA to the glucose treatment rescued the response impaired by glucose (p < 0.05, Figure 7C). In fact, 25-OH-D3 completely reversed heterophil respiratory burst suppressed by PA but not that caused by glucose alone or the glucose + PA treatment. Further, PA treatment promoted heterophil respiratory burst in the presence of glucose and 25-OH-D3 (p < 0.05, Figure 7C). In monocytes, glucose treatment impaired, but PA promoted respiratory burst response. Treatment with the combination of glucose and PA completely reversed the response impaired by glucose (p < 0.05, Figure 7C). Treatment with 25-OH-D3 significantly rescued the monocyte respiratory burst suppressed by glucose alone, but had no effects in the presence of PA. Interestingly, PA treatment significantly increased respiratory burst in both the glucose alone and glucose + 25-OH-D3 treatments (p < 0.05, Figure 7C).
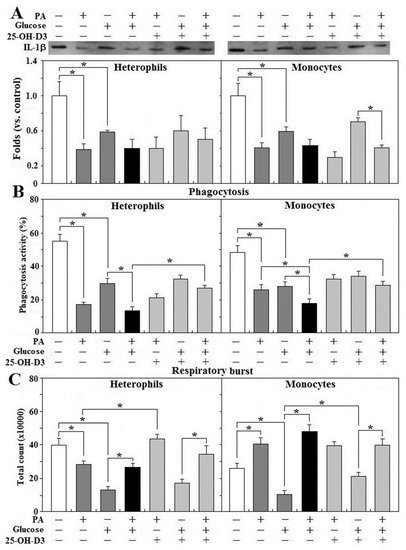
Figure 7. Effects of 25-OH-D3 on leukocyte functions challenged with glucose and/or palmitic acid. Peripheral heterophils and monocytes (2 × 106 cells) isolated from R-hens at age 61–65 weeks were treated with vehicle, 25-hydroxycholecalciferol (25-OH-D3, 50 nM), palmitic acid (PA, 1.5 mM), and/or glucose (200 mg/dL) overnight (16 h). Media were collected for interleukin-1β (IL-1β) analysis (A) based on the equivalent amounts of protein and cells used for phagocytosis (B) and respiratory burst analysis (C) (n = 4). Chemiluminescence results of respiratory burst analysis were expressed as integrated counts over 45 min. Results of Western blots were expressed as rations relative to control (no PA, glucose and 25-OH-D3 supplementation). *, significant difference vs. corresponding control (p < 0.05). + or − indicates with or without PA, glucose, or 25-OH-D3.
9. Mechanisms of Gluco/Lipotoxicity on Leukocyte Functions
In both types of cells, treatment with TC, a pharmacologic inhibitor of long chain fatty acyl-CoA synthetase that blocks fatty acid activation and downstream metabolism, FB1, the ceramide synthase inhibitor within the ceramide and sphingomyelin synthesis pathway, DPS, a functional inhibitor of acid sphingomyelinase that blocks sphingomyelin breakdown into ceramide, and nMPG that acts as a ROS scavenger, differentially rescued IL-1β secretion suppressed by PA or glucose (p < 0.05, Figure 8A). TC treatment exacerbated PA mediated suppression of phagocytosis in heterophils but not in monocytes, whereas FB1 partially rescued phagocytosis in both types of cells (p < 0.05, Figure 8B). Treatment with nMPG relieved phagocytosis impairment by PA or glucose in both types of leukocytes (p < 0.05, Figure 8B). In heterophils, DPS partially relieved respiratory burst suppressed by PA, whereas nMPG exacerbated PA or glucose mediated impairments (p < 0.05, Figure 8C). Treatment with TC or DPS reversed the PA-mediated increase in monocyte respiratory burst, while FB1 potentiated the increase by PA (p < 0.05, Figure 8C). nMPG significantly suppressed the promotion of respiratory burst by PA in monocytes to a level even lower than the control, and further exacerbated the suppression of respiratory burst by glucose (p < 0.05, Figure 8C).
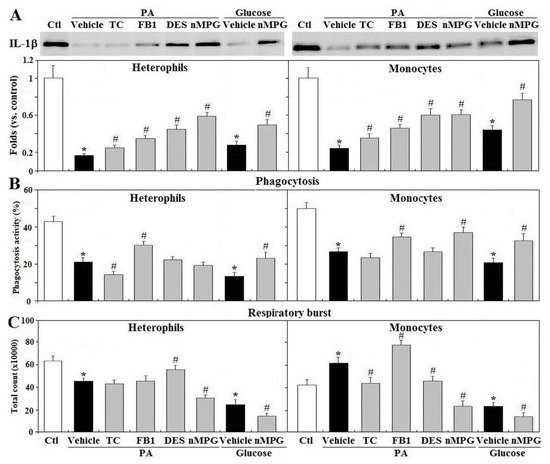
Figure 8. Mechanisms of glucolipotoxicity of leukocyte functions. Peripheral leukocytes (2 × 106 cells) from R-hens at age 61–65 weeks were pre-treated with TC (Triacsin C, 5 μM), FB1 (fumonisin B1, 25 μM), DPS (Desipramine, 10 μM), or nMPG (N-mercaptopropionyl-glycine, 0.3 mM). After being replaced with the medium, cells were treated with the vehicle, palmitic acid (PA, 1.5 mM), or glucose (200 mg/dL) overnight (16 h). Media were collected for interleukin-1β (IL-1β) analysis (A) by Western blot method based on equivalent amounts of protein and collected cells used for phagocytosis (B) and respiratory burst analysis (C) (n = 4). Chemiluminescence results of respiratory burst analysis were expressed as integrated counts over 45 min. Results of Western blots were expressed as rations relative to control (Ctl). *; significant difference vs. corresponding control (vehicle), p < 0.05, #; significant difference vs. vehicle, p < 0.05.
10. Effects of 25-OH-D3 on Leukocyte Viability Following Glucose or Palmitic Acid Challenge
In both types of leukocytes, treatment of glucose or PA suppressed cell viability in a dose-dependent manner and 25-OH-D3 partially rescued cell survival (p < 0.05, Figure 9A,B). However, 25-OH-D3 alone had no significant effects on cell viability in either cell type.
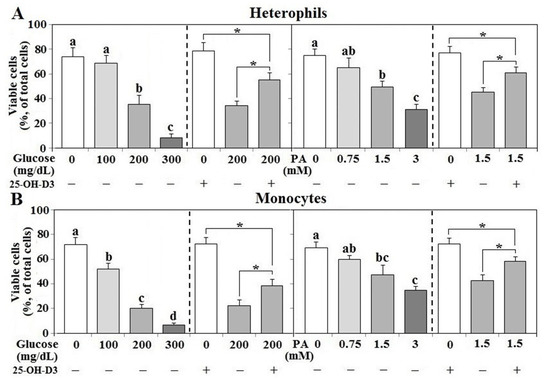
Figure 9. Effects of 25-OH-D3 on glucose or palmitic acid challenged leukocyte survival. Peripheral leukocytes (1 × 106 cells) isolated from R-hens at age 61–65 weeks were cultured with indicated levels of glucose or palmitic acid (PA) in the presence or absence of 25-hydroxycholecalciferol (25-OH-D3, 50 nM) overnight (16 h). Collected cell were used for cell death analysis (n = 4). Means with different letters differ significantly among different levels of glucose or PA (p < 0.05). Means with different letters differ significantly (p < 0.05). *, significant difference vs. corresponding control (p < 0.05). Means with different letters differ significantly (p < 0.05). + or − indicates with or without PA, glucose, or 25-OH-D3. Figure 9 is for cell death analysis, (A,B) mean different cell types, (A) heterophils, (B) mpnocytes.
References
- Griffin, H.D.; Goddard, C. Rapidly growing broiler (meat-type) chickens: Their origin and use for comparative studies of the regulation of growth. Int. J. Biochem. 1994, 26, 19–28.
- Yu, M.W.; Robinson, F.E.; Etches, R.J. Effect of feed allowance during rearing and breeding on female broiler breeders. 3. Ovarian steroidogenesis. Poult. Sci. 1992, 71, 1762–1767.
- Chen, C.Y.; Lin, H.Y.; Chen, Y.W.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Pathological adaption of cardiac hypertrophy. Poult. Sci. 2017, 96, 2428–2437.
- Chen, C.Y.; Huang, Y.F.; Ko, Y.J.; Liu, Y.J.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Obesity-associated cardiac pathogenesis in broiler breeder hens: Development of metabolic cardiomyopathy. Poult. Sci. 2017, 96, 2438–2446.
- Zou, A.; Nadeau, K.; Wang, P.W.; Lee, J.Y.; Guttman, D.S.; Sharif, S.; Korver, D.R.; Brumell, J.H.; Parkinson, J. Accumulation of genetic variants associated with immunity in the selective breeding of broilers. BMC Genet. 2020, 21, 5.
- Willson, N.L.; Forder, R.E.A.; Tearle, R.G.; Nattrass, G.S.; Hughes, R.J.; Hynd, P.I. Evaluation of fatty acid metabolism and innate immunity interactions between commercial broiler, F1 layer × broiler cross and commercial layer strains selected for different growth potentials. J. Anim. Sci. Biotechnol. 2017, 8, 70.
- Swaggerty, C.L.; Pevzner, I.Y.; Kaiser, P.; Kogut, M.H. Profiling pro-inflammatory cytokine and chemokine mRNA expression levels as a novel method for selection of increased innate immune responsiveness. Vet. Immunol. Immunopathol. 2008, 126, 35–42.
- Cheema, M.A.; Qureshi, M.A.; Havenstein, G.B. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1519–1529.
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of adaptive and innate immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269.
- Lee, C.H.; Lam, K.S. Obesity-induced insulin resistance and macrophage infiltration of the adipose tissue: A vicious cycle. J. Diabetes Investig. 2019, 10, 29–31.
- Richardson, V.R.; Smith, K.A.; Carter, A.M. Adipose tissue inflammation: Feeding the development of type 2 diabetes mellitus. Immunobiology 2013, 218, 1497–1504.
- Liu, Z.C.; Xie, Y.L.; Chang, C.J.; Su, C.M.; Chen, Y.H.; Huang, S.Y.; Walzem, R.L.; Chen, S.E. Feed intake alters immune cell functions and ovarian infiltration in broiler hens: Implications for reproductive performance. Biol. Reprod. 2014, 90, 1–8.
- Xie, Y.L.; Pan, Y.E.; Chang, C.J.; Tang, P.C.; Huang, Y.F.; Walzem, R.L.; Chen, S.E. Palmitic acid in chicken granulosa cell death-lipotoxic mechanisms mediate reproductive inefficacy of broiler breeder hens. Theriogenology 2012, 78, 1917–1928.
- Pan, Y.E.; Liu, Z.C.; Chang, C.J.; Xie, Y.L.; Chen, C.Y.; Chen, C.F.; Walzem, R.L.; Chen, S.E. Ceramide accumulation and up-regulation of proinflammatory interleukin-1β exemplify lipotoxicity to mediate declines of reproductive efficacy of broiler hens. Domest. Anim. Endocrinol. 2012, 42, 183–194.
- Huang, Y.F.; Chang, L.C.; Chen, C.Y.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Unrestricted Feed Intake Induces β-Cell Death and Impairs Insulin Secretion in Broiler Breeder Hens. Animals 2020, 10, 1969.
- Chen, L.; Eapen, M.S.; Zosky, G.R. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci. Rep. 2017, 7, 45172.
- Ambrose, C.T. The Osler slide, a demonstration of phagocytosis from 1876 Reports of phagocytosis before Metchnikoff’s 1880 paper. Cell. Immunol. 2006, 240, 1–4.
- Vanlint, S. Vitamin D and obesity. Nutrients 2013, 5, 949–956.
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Dekker, J.M.; Marz, W. Vitamin D deficiency and myocardial diseases. Mol. Nutr. Food Res. 2010, 54, 1103–1113.
- Hewison, M. Vitamin D and the intracrinology of innate immunity. Mol. Cell. Endocrinol. 2010, 321, 103–111.
- Teymoori-Rad, M.; Shokri, F.; Salimi, V.; Marashi, S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019, 29, e2032.
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625.
- Geng, Y.; Ma, Q.; Wang, Z.; Guo, Y. Dietary vitamin D3 supplementation protects laying hens against lipopolysaccharide-induced immunological stress. Nutr. Metab. 2018, 15, 58.
- Chou, P.C.; Chen, Y.H.; Chung, T.K.; Walzem, R.L.; Lai, L.S.; Chen, S.E. Supplemental 25-hydroxycholecalciferol Alleviates Inflammation and Cardiac Fibrosis in Hens. Int. J. Mol. Sci. 2020, 21, 8379.
- Lin, H.Y.; Chung, T.K.; Chen, Y.H.; Walzem, R.L.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol improves livability in broiler breeder hens. Poult. Sci. 2019, 98, 6108–6116.
- Lin, H.Y.; Chou, P.C.; Chen, Y.H.; Lai, L.S.; Chung, T.K.; Walzem, R.L.; Huang, S.Y.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol Improves livability in broiler breeder hens-amelioration of cardiac pathogenesis and hepatopathology. Animals 2019, 9, 770.
- Yeh, Y.L.; Chou, P.C.; Chen, Y.H.; Lai, L.S.; Chung, T.K.; Walzem, R.L.; Huang, S.Y.; Chen, S.E. Dietary supplementation of 25-hydroxycholecalciferol improves cardiac function and livability in broiler breeder hens–amelioration of blood pressure and vascular remodeling. Poult. Sci. 2020, 99, 3363–3373.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
717
Revisions:
2 times
(View History)
Update Date:
18 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




