
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Evangelia Tsiani | + 2306 word(s) | 2306 | 2021-06-11 05:07:23 | | | |
| 2 | Lindsay Dong | Meta information modification | 2306 | 2021-06-17 03:20:49 | | |
Video Upload Options
Regular exercise/physical activity is beneficial for the health of an individual and lowers the risk of getting different diseases, including cancer. How exactly exercise results in these health benefits is not known. Recent studies suggest that the molecule irisin released by muscles into the blood stream after exercise may be responsible for these effects.
1. Introduction
Recent evidence indicates that the exercising muscle releases proteins called myokines into the bloodstream, allowing them to be delivered to different tissues in the body and exert the beneficial effects of exercise (Figure 1). Prominent myokines produced by skeletal muscle are interleukins-6 and -15 (IL-6 and IL-15), oncostatin, myostatin, brain-derived neurotrophic factor (BDNF) and irisin [1][2][3].
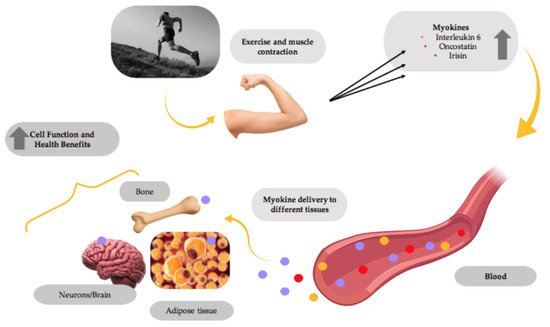
Figure 1. Myokines mediate the health benefits of exercise. Muscle contractions induce the production and release of myokines by muscle, which are delivered through the blood circulation to different tissues in the body, improving the overall tissue function and providing health benefits. This figure was created with BioRender.com.
2. Irisin
2.1. Irisin Structure and Synthesis
Irisin is part of the fibronectin type III domain containing 5 (FNDC5) protein. The human FNDC5 gene has a different start codon (ATA) compared to other species such as the mouse or rat (ATG) [4], and this ATA start codon is associated with a low expression efficiency [5].
Muscle cells produce fibronectin type III domain containing 5 (FNDC5), a protein whose destination is to be localized to the plasma membrane. The original FNDC5 protein contains an N-terminal signal sequence (Figure 2), which targets it to the plasma membrane, and is subsequently cleaved, as is true for all signal peptide sequences of plasma membrane proteins. The N-terminal signal sequence is followed by a fibronectin type III domain (FNIII), a transmembrane domain and a c-terminal tail corresponding to the cytosolic region of the protein (Figure 2). Irisin is produced following the proteolytic cleavage of the mature FNDC5 protein (Figure 2).
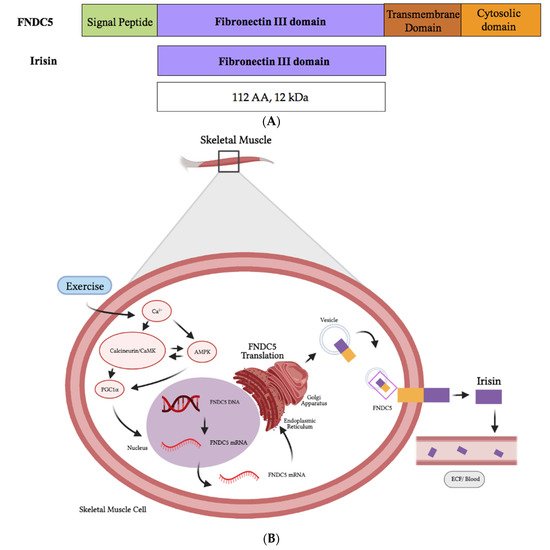
Figure 2. Schematic representation of FNDC5 and the irisin protein domains (A). The synthesis and release of irisin by muscle cells is induced by exercise (B). Figure 2B was created with BioRender.com.
2.2. Irisin Blood Levels, Clearance and Tissue Distribution
The measurements indicated that, in a sedentary individual, the irisin levels are at approximately 3.6 ng/mL, while the levels found in individuals after exercise are much higher at ~4.3 ng/mL [6]. Similarly, Moreno et al. [7] found higher circulating irisin levels in physically active individuals compared to sedentary subjects.
Overall, the existing data provided evidence that irisin is released in response to exercise. The half-life of irisin was examined in vivo by Kim et al. in mice injected with recombinant irisin for 6 days and was found to be less than an hour [8]. If these data extend to humans, and the half-life of endogenously produced irisin in humans is also less than an hour, they could contribute to the variability seen in the blood/circulating irisin levels. Apart from exercise, cold exposure and various drugs such as statins, metformin and phytochemicals/polyphenols such as resveratrol and citrus flavonoids, as well as diet, could all influence the blood irisin levels [9].
The tissue distribution and clearance of irisin in vivo has not been extensively studied. Lv et al. administered radioactively labelled irisin by injection in mice and utilized single-photon emission computerized tomography (SPECT)/CT imagining to examine irisin distribution in different tissues. The highest level of irisin was found in the gallbladder, followed by the liver and kidneys [10]. It was also found that the clearance of irisin is through the hepato-biliary and the renal system [10].
2.3. Irisin receptor and Mechanism of Action in Target Tissues
Currently, there is no irisin receptor identified. Recent studies have shown that, in osteocytes, irisin binds to the αV/β5 integrin receptor complex and activates the focal adhesion kinase (FAK), a well-established downstream signaling molecule of a αV/β5 integrin receptor, while the use of αV/β5 integrin receptor inhibitors blocked the irisin effects [8]. Similarly, using the immunofluorescence analysis, it was shown in vitro and in vivo that irisin binds to the integrin αVβ5 receptor on gut epithelial cells [11][12]. Wei et al. [13] found that the use of integrin (αV) inhibitors (RGD peptide, Echistain) blocked the irisin effects on hepatocytes (L02), proving further evidence of irisin signaling via integrin receptor binding. These recent studies provided the initial evidence of the binding of irisin to αVβ5 integrin complexes. However, it is possible that irisin does not bind solely on this complex and may bind to other members of integrins or other membrane receptors. Further studies are needed in the future for a better understanding of irisin receptors.
3. Role of Irisin in Cancer
3.1. Role of Irisin in Cancer: In Vitro Evidence
Limited studies (presented below) have examined the direct effects of irisin on cancer cells. The exposure of endometrial (KLE and RL95-2), colon (HT2 and, MCA38), thyroid (SW579 and BHP7) and esophageal (OE13 and OE33) cancer cells to physiological (5–10 nmol/L) and high physiological/pharmacological (50–100 nmol/L) concentrations of irisin did not affect cell adhesion and colony formation [14] (Table 1). These data indicated no changes in the proliferation and malignant potential of cancer cells within irisin treatment.
Table 1. Role of irisin in cancer: in vitro evidence.
| Cancer Cell | Irisin Concentration/Duration | Findings | Reference |
|---|---|---|---|
| KLE, RL95-2 endometrial cancer HT29, MCA38 colon cancer SW579, BHP7 thyroid cancer OE13, OE33 esophageal cancer |
5, 10, 50, 100 nM/36 h | no effect on cell adhesion no effect on colony formation |
[14] |
| MDA-MB-231 breast cancer |
0.625–20 nM/24 h | ↓ cell viability ↓ cell migration ↓ NF-κB ↑ caspase 3/7cleavage ↑ apoptosis |
[15] |
| LNCaP (androgen receptor positive) prostate cancer DU-145, PC3 (androgen receptor negative) prostate cancer |
0.1, 1, 10 and 100 nM/24 h | ↓ proliferation (cell viability) | [16] |
| A549, NCI-H446 lung cancer |
20 nM/24 h | ↓ proliferation ↓ migration ↓ invasion ↓ EMT ↓ PI3K/AKT ↓ Snail |
[17] |
| A549, H1299, H358, H1650 lung cancer |
20 nM/24–96 h | ↓ proliferation ↓ MDR1 ↓ NF-κB |
[18] |
| U2OS, MG-63 osteosarcoma |
100 ng/mL/24 h | ↓ proliferation ↓ migration ↓ invasion ↓ EMT ↓ STAT3 ↓ Snail |
[19] |
| U2OS osteosarcoma |
25, 50, 100, 200 ng/mL | ↓ proliferation ↓ migration ↓ EMT ↓ invasion |
[20] |
| HepG2, SMCC7721 hepatocellular carcinoma |
2.5 nM/24 h | ↑ proliferation ↑ migration ↑ invasion ↑ PI3K |
[21] |
| MIA PaCa-2, Panc03.27 pancreatic cancer |
10 and 100 nM/24 h | ↓ proliferation ↓ colony formation ↓ migration ↑ cell cycle arrest (G1) ↓ EMT ↓ vimentin ↑ E-cadherin ↑ AMPK activation ↓ mTOR activation |
[22] |
| MIA PaCa-2, BxPC-3, Panc03.27 pancreatic cancer |
5, 10, 50, 100 nM/24 h | ↑ apoptosis ↑ PARP cleavage ↑ caspase-3 cleavage ↓ BCL-2 ↓ BCL-xL ↓ Akt ↓ NF-κB |
[23] |
| PANC-1,BxPC-3 pancreatic cancer |
0, 10, 20 and 50 nM/24 h | ↓ proliferation ↑ apoptosis ↓ migration ↓ invasion ↓ Akt |
[24] |
| PANC-1 pancreatic cancer |
100 nM/12 h | ↑ erastin-induced apoptosis ↑ ROS levels ↓ GSH levels ↓ NRF2 ↓ P62 |
[25] |
| U-87 MG, T98G, LN-18 glioblastoma |
1 μM/72 h | ↓ proliferation ↑ cell cycle arrest ↑ p21 mRNA, protein ↓ invasion ↑ TFPI-2 mRNA, protein |
[26] |
Overall, the evidence from the majority of the available in vitro studies indicates the inhibition of proliferation, survival, migration and invasion and the induction of apoptosis of cancer cells exposed to irisin (Table 1). These anticancer effects of irisin were associated with the inhibition of PI3K, Akt, mTOR, STAT3 and NF-κB and activation of AMPK (Figure 3). It is not known if the inhibition of PI3K, Akt, BCL-2, NF-kB, STAT3 and mTOR by irisin is direct or indirect. It is possible that irisin directly inhibits them or indirectly modulates the upstream regulators. For example, the inhibition of mTOR may be due to the activation of the cellular energy sensor AMPK, an upstream regulator (inhibitor) of mTOR.
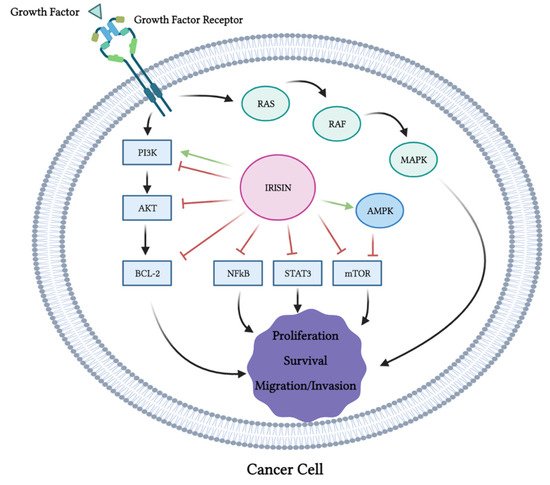
Figure 3. Effects of irisin on cancer cell signaling molecules. PI3K, Akt, Bcl-2, NF-κB, STAT3 and mTOR were all inhibited by irisin. Irisin activated AMPK and MAPK. This figure is based on the data of the studies mentioned in the in vitro section and Table 1. The figure was created with BioRender.com.
The levels of cleaved caspase 3, 7 and PARP, all markers of apoptosis, were enhanced with the irisin treatment (Figure 3). It is important to note that one study showed no effects of irisin on cancer cell proliferation [14], while another study utilizing hepatocellular carcinoma cells showed increased proliferation, migration and invasion with irisin treatment [21] that was associated with activation of the PI3K/Akt signaling pathway. The existence of these contradictory evidence point to the requirement of more research and in-depth investigation of the biological effects and the role of irisin in tissue homeostasis. It may also suggest that the effects of irisin are cell- and tissue-specific.
3.2. Role of Irisin in Cancer: In Vivo Evidence
3.2.1. Animal Models of Cancer
Altay et al. examined the levels of irisin in control BALB/c mice and in mice with gastric tumors induced by the administration of N-nitroso-N-methylurea (MNU) [27] (Table 2). The serum levels of the markers of inflammation and cachectic factors were elevated in mice with induced cancer compared to the control animals. FNDC5 and the cachectic factor zinc-α-2 glycoprotein were not detected in gastric tissues in any animal group.
Table 2. Role of Irisin in cancer: in vivo evidence from animal studies.
| Animal Model | Intervention (Treatment) | Findings | Reference |
|---|---|---|---|
| BALB/c mice | N-nitroso-N-methylurea (MNU) to induce gastric cancer | ↑ FNDC5 mRNA levels in white and brown adipose tissue of mice in pre-cancer and cancer groups ↑ Irisin levels in mice of pre-cancer and cancer groups |
[27] |
| Athymic male nude mice injected with human glioblastoma cells (U-87 MG) |
irisin 20 μg/day, 14 days | ↓ reduced tumor volume | [26] |
3.2.2. Human Studies
The serum irisin levels were found significantly lower in female patients with invasive ductal breast cancer compared with the control healthy women [28] (Table 3). A significant independent association between the serum irisin levels and development of breast cancer was found in these female patients. There was an estimation that a 1-μg unit of increase in the irisin levels results in almost a 90% decrease in the risk of breast cancer development.
Table 3. Role of irisin in cancer: in vivo evidence from human studies measuring the serum irisin levels.
| Participants | Measurements | Findings | Reference |
|---|---|---|---|
| Healthy humans, breast cancer patients (101 invasive ductal) N = 152 |
serum irisin levels (ELISA) | ↓ serum irisin levels in breast cancer patients | [28] |
| Breast cancer patients (+ spinal metastasis) N = 148 |
serum irisin levels (ELISA) | ↓ serum irisin levels in breast cancer patients with spinal metastasis | [29] |
| Hepatocellular carcinoma patients N = 36 |
serum irisin levels (ELISA) | no significant difference in serum irisin levels in HCC patients vs. donors | [30] |
| Patients with hepatocellular carcinoma N = 20 |
serum irisin levels (ELISA) | no significant difference in serum irisin levels | [21] |
| Healthy humans, hepatocellular carcinoma patients N = 219 |
serum irisin levels (ELISA) | ↓ serum irisin levels in HCC patients ↓ FNDC5/irisin levels in HCC tissues |
[31] |
| Healthy humans, hepatocellular carcinoma patients N = 43 |
serum irisin levels (ELISA) | ↓ serum irisin levels in HCC patients ↓ FNDC5/irisin levels in HCC tissues |
[32] |
| Colorectal cancer patients Obese and non-obese N = 116 |
serum irisin levels (ELISA) | ↓ serum irisin levels in CRC patients | [33] |
| Renal cancer patients N = 48 |
serum irisin levels (ELISA) | ↑ serum irisin levels in renal cancer patients | [34] |
| Healthy humans, newly diagnosed bladder cancer patients N = 150 |
serum irisin levels (ELISA) | ↓ serum irisin levels in bladder cancer patients | [35] |
| Healthy humans, prostate cancer patients N = 80 |
serum irisin levels (ELISA) | ↓ serum irisin levels in prostate cancer patients | [36] |
| Healthy humans, gastric cancer patients N = 51 |
serum irisin levels (ELISA) | ↑ serum irisin levels in gastric cancer patients | [37] |
| Healthy humans, breast cancer patients N = 213 |
serum irisin levels (ELISA) | ↑ serum irisin levels in both benign and malignant breast tumor cases compared to control | [38] |
Aydin et al. examined the levels of irisin in various cancer tissues by IHC utilizing an irisin antibody and compared them to the levels found in control healthy tissues [39] (Table 4). All the tissues were from patients who received no chemotherapy or radiotherapy before their operations. Irisin was found in most of the tissues examined (brain, esophageal, stomach, liver, colon and pancreas), with significantly increased levels seen in gastrointestinal cancer and grade II astrocytoma tissues compared to the control [39].
Table 4. Role of irisin in cancer: in vivo evidence from human studies measuring irisin in cancer tissues.
| Participants | Measurements | Findings | Reference |
|---|---|---|---|
| Healthy humans, tumor tissues (brain, esophagus, stomach liver, pancreas) N = N/A |
irisin expression in healthy and cancer tissues (IHC) | ↑ irisin levels in gastrointestinal cancer, grade II astrocytoma | [39] |
| Healthy humans, tumor tissues (breast, cervix, ovaries, endometrium) N = N/A |
irisin expression in healthy and cancer tissues (IHC) | ↑ irisin levels in breast, ovarian, cervical and endometrial tumor tissues | [40] |
| Hepatocellular carcinoma patients N = 36 |
FNDC5 mRNA levels measured in liver tissues of HCC patients and controls (RT-PCR) |
↑ FNDC5/irisin hepatic mRNA levels | [30] |
| Healthy humans, patients with hepatocellular carcinoma N = 20 |
FNDC5 mRNA levels measured in liver tissues of HCC patients and controls (RT-PCR) |
↑ FNDC5 mRNA levels in HCC patients compared to controls | [21] |
| Healthy humans, hepatocellular carcinoma patients N = 219 |
FNDC5/irisin expression in HCC tissues | ↓ FNDC5/irisin levels in HCC tissues | [31] |
| Healthy humans, hepatocellular carcinoma patients N = 43 |
FNDC5/irisin expression in HCC tissues | ↓ FNDC5/irisin levels in HCC tissues | [32] |
| Colorectal cancer patients obese and non-obese N = 116 |
FNDC5/irisin levels in subcutaneous and visceral white adipose tissues (RT-PCR) | no difference between FNDC5 levels in subcutaneous and visceral white adipose tissues. | [33] |
| Renal cancer patients N = 110 |
irisin expression in healthy and cancer tissues (IHC) | ↓ FNDC5/irisin in chromophobe renal cell carcinoma | [41] |
| Healthy humans, thyroid cancer patients N = 160 |
irisin expression in healthy and cancer tissues (IHC) | ↑ irisin in oncocytic papillary carcinoma ↑ irisin in anaplastic carcinoma |
[42] |
| Non-small cell lung cancer patients N = 729 |
FNDC5/irisin expression in cancer tissues (IHC and RT-PCR) | ↓ FNDC5/irisin levels in NSCLC tissue ↑ FNDC5/irisin levels in stromal fibroblasts |
[43] |
4. Conclusions
The majority of the available in vitro studies indicate the inhibition of cancer cell proliferation, survival and migration with irisin treatment. However, the effects of irisin in human and animal cells in culture may be different from the effects seen in vivo. Studies from breast [28][29], hepatocellular [31], colorectal [33], renal [34], prostate [36] and gastric [37] cancer patients suggest that the serum irisin levels may serve as a diagnostic marker. Some in vivo studies have found increased irisin levels in various cancerous tissues, while others have shown the opposite. Furthermore, it is not clear whether the altered expression of irisin seen in tumor tissue is the cause of tumorigenesis or a compensatory mechanism to counteract tumorigenesis.
Currently, controversies exist surrounding the detection of the irisin gene and protein expression in tissues and hopefully these controversies will soon be resolved. Irisin expression in different cancer tissues should be studied extensively, and its role in tumorigenesis should be elucidated before irisin can be used for cancer diagnosis, prognosis and/or treatment. Clearly, more in vivo animal and human studies are required.
References
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin--Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, 320724.
- So, B.; Kim, H.-J.; Kim, J.; Song, W. Exercise-Induced Myokines in Health and Metabolic Diseases. Integr. Med. Res. 2014, 3, 172–179.
- Di Raimondo, D.; Miceli, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A. New Insights about the Putative Role of Myokines in the Context of Cardiac Rehabilitation and Secondary Cardiovascular Prevention. Ann. Transl. Med. 2017, 5, 300.
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T.; et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 2013, 8, e73680.
- Kozak, M. Context Effects and Inefficient Initiation at Non-AUG Codons in Eucaryotic Cell-Free Translation Systems. Mol. Cell. Biol. 1989, 9, 5073–5080.
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740.
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100.
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17.
- Flori, L.; Testai, L.; Calderone, V. The “Irisin System”: From Biological Roles to Pharmacological and Nutraceutical Perspectives. Life Sci. 2021, 267, 118954.
- Lv, J.; Pan, Y.; Li, X.; Cheng, D.; Ju, H.; Tian, J.; Shi, H.; Zhang, Y. Study on the Distribution and Elimination of the New Hormone Irisin in Vivo: New Discoveries Regarding Irisin. Horm. Metab. Res. 2015, 47, 591–595.
- Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Darkwah, S.; Kawamoto, E.; Shimaoka, M. Integrin-Ligand Interactions in Inflammation, Cancer, and Metabolic Disease: Insights Into the Multifaceted Roles of an Emerging Ligand Irisin. Front. Cell Dev. Biol. 2020, 8, 588066.
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wu, Z.; Lv, Y.; et al. Irisin Reverses Intestinal Epithelial Barrier Dysfunction during Intestinal Injury via Binding to the Integrin AVβ5 Receptor. J. Cell. Mol. Med. 2020, 24, 996–1009.
- Wei, S.; Bi, J.; Yang, L.; Zhang, J.; Wan, Y.; Chen, X.; Wang, Y.; Wu, Z.; Lv, Y.; Wu, R. Serum Irisin Levels Are Decreased in Patients with Sepsis, and Exogenous Irisin Suppresses Ferroptosis in the Liver of Septic Mice. Clin. Transl. Med. 2020, 10, e173.
- Moon, H.-S.; Mantzoros, C.S. Regulation of Cell Proliferation and Malignant Potential by Irisin in Endometrial, Colon, Thyroid and Esophageal Cancer Cell Lines. Metabolism 2014, 63, 188–193.
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202.
- Tekin, S.; Erden, Y.; Sandal, S.; Yilmaz, B. Is Irisin an Anticarcinogenic Peptide? Med. Sci. Int. Med. J. 2015, 4, 2172.
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605.
- Fan, G.-H.; Zhu, T.-Y.; Huang, J. FNDC5 Promotes Paclitaxel Sensitivity of Non-Small Cell Lung Cancers via Inhibiting MDR1. Cell. Signal. 2020, 72, 109665.
- Kong, G.; Jiang, Y.; Sun, X.; Cao, Z.; Zhang, G.; Zhao, Z.; Zhao, Y.; Yu, Q.; Cheng, G. Irisin Reverses the IL-6 Induced Epithelial-Mesenchymal Transition in Osteosarcoma Cell Migration and Invasion through the STAT3/Snail Signaling Pathway. Oncol. Rep. 2017, 38, 2647–2656.
- Cheng, G.; Xu, D.; Chu, K.; Cao, Z.; Sun, X.; Yang, Y. The Effects of MiR-214-3p and Irisin/FNDC5 on the Biological Behavior of Osteosarcoma Cells. Cancer Biother. Radiopharm. 2020, 35, 92–100.
- Shi, G.; Tang, N.; Qiu, J.; Zhang, D.; Huang, F.; Cheng, Y.; Ding, K.; Li, W.; Zhang, P.; Tan, X. Irisin Stimulates Cell Proliferation and Invasion by Targeting the PI3K/AKT Pathway in Human Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 2017, 493, 585–591.
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin Inhibits Pancreatic Cancer Cell Growth via the AMPK-MTOR Pathway. Sci. Rep. 2018, 8, 15247.
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-ΚB Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6085–6096.
- Zhang, D.; Zhang, P.; Li, L.; Tang, N.; Huang, F.; Kong, X.; Tan, X.; Shi, G. Irisin Functions to Inhibit Malignant Growth of Human Pancreatic Cancer Cells via Downregulation of the PI3K/AKT Signaling Pathway. OncoTargets Ther. 2019, 12, 7243–7249.
- Yang, B.C.; Leung, P.S. Irisin Is a Positive Regulator for Ferroptosis in Pancreatic Cancer. Mol. Ther. Oncolytics 2020, 18, 457–466.
- Huang, C.-W.; Chang, Y.-H.; Lee, H.-H.; Wu, J.-Y.; Huang, J.-X.; Chung, Y.-H.; Hsu, S.-T.; Chow, L.-P.; Wei, K.-C.; Huang, F.-T. Irisin, an Exercise Myokine, Potently Suppresses Tumor Proliferation, Invasion, and Growth in Glioma. FASEB J. 2020, 34, 9678–9693.
- Altay, D.U.; Keha, E.E.; Ozer Yaman, S.; Ince, I.; Alver, A.; Erdogan, B.; Canpolat, S.; Cobanoglu, U.; Mentese, A. Investigation of the Expression of Irisin and Some Cachectic Factors in Mice with Experimentally Induced Gastric Cancer. QJM Int. J. Med. 2016, 109, 785–790.
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer 2015, 15, 898.
- Zhang, Z.-P.; Zhang, X.-F.; Li, H.; Liu, T.-J.; Zhao, Q.-P.; Huang, L.-H.; Cao, Z.-J.; He, L.-M.; Hao, D.-J. Serum Irisin Associates with Breast Cancer to Spinal Metastasis. Medicine (Baltimore) 2018, 97, e0524.
- Gaggini, M.; Cabiati, M.; Del Turco, S.; Navarra, T.; De Simone, P.; Filipponi, F.; Del Ry, S.; Gastaldelli, A.; Basta, G. Increased FNDC5/Irisin Expression in Human Hepatocellular Carcinoma. Peptides 2017, 88, 62–66.
- Zhang, J.; Ke, M.; Ren, Y.; Bi, J.; Du, Z.; Zhang, M.; Wang, Y.; Zhang, L.; Wu, Z.; Lv, Y.; et al. Serum Irisin Predicts Posthepatectomy Complications in Patients with Hepatocellular Carcinoma. Dis. Markers 2019, 2019, 9850191.
- Pazgan-Simon, M.; Zuwała-Jagiełło, J.; Kukla, M.; Grzebyk, E.; Simon, K. Serum Concentrations of Selected Adipokines in Virus-Related Liver Cirrhosis and Hepatocellular Carcinoma. Clin. Exp. Hepatol. 2020, 6, 235–242.
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue MRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients With or Without Obesity. Front. Physiol. 2018, 9, 1125.
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2018, 44, 734–739.
- Esawy, M.M.; Abdel-Samd, K.M. The Diagnostic and Prognostic Roles of Serum Irisin in Bladder Cancer. Curr. Probl. Cancer 2020, 44, 100529.
- Aslan, R.; Alp, H.H.; Eryılmaz, R.; Huyut, Z.; Sevim, M.; Araz, Ş.; Ertas, K.; Taken, K. Can the Irisin Be a Biomarker for Prostate Cancer? A Case Control Study. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 505–509.
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating Irisin Levels and Redox Status Markers in Patients with Gastric Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 2847–2851.
- Panagiotou, G.; Triantafyllidou, S.; Tarlatzis, B.C.; Papakonstantinou, E. Serum Levels of Irisin and Omentin-1 in Breast Neoplasms and Their Association with Tumor Histology. Int. J. Endocrinol. 2021, 2021, 6656671.
- Aydin, S.; Kuloglu, T.; Ozercan, M.R.; Albayrak, S.; Aydin, S.; Bakal, U.; Yilmaz, M.; Kalayci, M.; Yardim, M.; Sarac, M.; et al. Irisin Immunohistochemistry in Gastrointestinal System Cancers. Biotech. Histochem. 2016, 91, 242–250.
- Kuloglu, T.; Celik, O.; Aydin, S.; Hanifi Ozercan, I.; Acet, M.; Aydin, Y.; Artas, G.; Turk, A.; Yardim, M.; Ozan, G.; et al. Irisin Immunostaining Characteristics of Breast and Ovarian Cancer Cells. Cell. Mol. Biol. 2016, 62, 40–44.
- Kuloğlu, T.; Artaş, G.; Yardim, M.; Sahin, I.; Aydin, Y.; Beyoğlu, N.; Özercan, I.H.; Yalcin, M.H.; Ugur, K.; Aydin, S. Immunostaining Characteristics of Irisin in Benign and Malignant Renal Cancers. Biotech. Histochem. 2019, 94, 435–441.
- Ugur, K.; Aydin, S.; Kuloglu, T.; Artas, G.; Kocdor, M.A.; Sahin, İ.; Yardim, M.; Ozercan, İ.H. Comparison of Irisin Hormone Expression between Thyroid Cancer Tissues and Oncocytic Variant Cells. Cancer Manag. Res. 2019, 11, 2595–2603.
- Nowinska, K.; Jablonska, K.; Pawelczyk, K.; Piotrowska, A.; Partynska, A.; Gomulkiewicz, A.; Ciesielska, U.; Katnik, E.; Grzegrzolka, J.; Glatzel-Plucinska, N.; et al. Expression of Irisin/FNDC5 in Cancer Cells and Stromal Fibroblasts of Non-Small Cell Lung Cancer. Cancers 2019, 11, 1538.




