Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federica Maggi | + 1711 word(s) | 1711 | 2021-06-03 05:49:30 | | | |
| 2 | Lily Guo | Meta information modification | 1711 | 2021-06-13 05:01:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maggi, F. TRP channels in hematological malignancies. Encyclopedia. Available online: https://encyclopedia.pub/entry/10790 (accessed on 07 February 2026).
Maggi F. TRP channels in hematological malignancies. Encyclopedia. Available at: https://encyclopedia.pub/entry/10790. Accessed February 07, 2026.
Maggi, Federica. "TRP channels in hematological malignancies" Encyclopedia, https://encyclopedia.pub/entry/10790 (accessed February 07, 2026).
Maggi, F. (2021, June 11). TRP channels in hematological malignancies. In Encyclopedia. https://encyclopedia.pub/entry/10790
Maggi, Federica. "TRP channels in hematological malignancies." Encyclopedia. Web. 11 June, 2021.
Copy Citation
Transient receptor potential (TRP) channels are improving their importance in different cancers, becoming suitable as promising candidates for precision medicine. In particular, this section will be focused on TRP importance in hematological malignancies.
TRP
1. General introduction to TRP
Transient receptor potential (TRP) channels are a family of ion channels, belonging to voltage-gated superfamilies, with different physiological functions [1][2][3]. TRP channels are expressed in many organisms, from worms to mammals, and grouped in six subfamilies based on their amino acid sequence homology: canonical (TRPC), vanilloid (TRPV), melastatin (TRPM), ankyrin (TRPA), polycystic (TRPP), mucolipin (TRPML) (Figure 1) [4]. Most of TRPs are selective for cations and most of them are permeable to both monovalent cations, such as sodium (Na+), and divalent cations, such as magnesium (Mg2+) or calcium (Ca2+) [5]. Moreover, they can be activated by a variety of environmental stimuli such as temperature (as TRPV2), mechanical forces (as TRPA1), or plant-derived compounds such as menthol for TRPM8 or capsaicin (CPS) for TRPV1 (Table 1) [1][3][6].
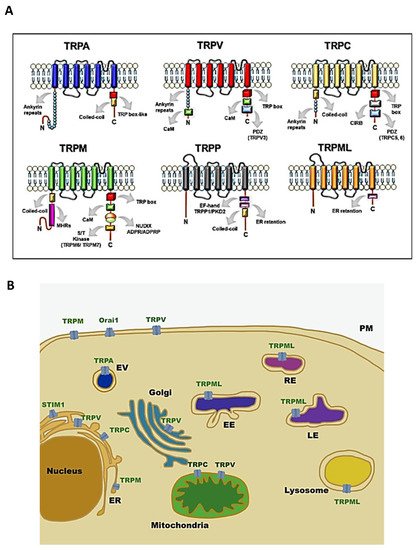
Figure 1. Structure and localisation of TRP channels: (A) structural domains and motifs in the N- and C- terminus of TRP channel subfamilies [7]; (B) intracellular localisation of TRP channel subfamilies and SOCE machinery. Abbreviations: TRPA, transient receptor potential ankyrin; TRPC, transient receptor potential canonical; TRPM, transient receptor potential melastatin; TRPML, transient receptor potential mucolipidin; TRPP, transient receptor potential polycystic; TRPV, transient receptor potential vanilloid; ER, endoplasmatic reticulum; EV, vesicle of exocytosis; EE, early endosome; RE, recycling endosome; LE, late endosome; PM, plasma membrane; STIM1, stromal interaction molecule 1; Orai1, calcium release-activated calcium channel protein 1.
Table 1. Environmental activating stimuli and calcium permeability characteristics of TRP channels.
| TRP | Ca2+ Selectivity | Activation Temperature | Exogenous Activators | References |
|---|---|---|---|---|
| TRPA1 | Medium/Low | <17 °C | Caffeine, Cinnamaldehyde, Nicotine | [8][9][10] |
| TRPC1 | Medium | Lanthanide ions (La3+, Gd3+), carbachol | [11] | |
| TRPC3 | Medium | OAG | [12] | |
| TRPC6 | Medium | OAG, Hyperforin, 2,4-Diacylphloroglucinol | [12][13][14] | |
| TRPM2 | Medium/Low | >38 °C | N-(p-amylcinnamoyl)anthranilic acid, Clotrimazole, Flufenamic acid | [15][16][17][18] |
| TRPM4 | Non selective | 15–35 °C | BTP2 | [15][19][20] |
| TRPM7 | Medium/Low | Naltriben | [21] | |
| TRPM8 | Medium | 17–25 °C | Menthol, eucalyptol, geraniol | [6] |
| TRPML2 | Non selective | SID24801657, SID24787221, ML2SA1 | [22][23] | |
| TRPV1 | Medium/High | >42 °C | CPS, Piperine, Gingerol | [6][24] |
| TRPV2 | Medium | (≈52 °C) | 2-aminoethoxydiphenyl borate, diphenylyboronic anhydride, and Cannabis sativa derivatives | [6] |
| TRPV4 | Medium | 24–27 °C | Bisandrographolide A, Apigenin, 4-alpha-phorbol12,13-didecanoate | [6] |
| TRPV5 | High | - | ||
| TRPV6 | High | CPS | [25] |
Initially, it was thought that TRP channels were solely expressed on plasma membrane (PM) mediating cation entry, but many studies have demonstrated that almost all mammalian TRP channels are located in the intracellular vesicular membranes (Figure 1B). It is unclear whether TRPs are expressed in these compartments as intermediate biosynthetic pathways and then shuttled to their final location, or if they have a role as signal transducers and/or membrane trafficking regulators [26].
Above all, they are considered important Ca2+ selective ion channels (Table 1) which have been linked to countless physiological and pathological functions [27].
Indeed, calcium homeostasis shows a precise and fine regulation, and it constitutes the core of all cellular signals. Thus far, many studies have shown that cytosolic Ca2+ oscillations play a pivotal role in the carcinogenesis process. Tumour cells are able to remodel the Ca2+ signalling network and its disruption contributes to malignant phenotype development. The connection between Ca2+ signalling and cancer is an interesting field of study, and the emergent role of ion channels such as TRPs in regulating the Ca2+ network makes them promising targets for cancer treatment and care [27][28][29].
2. TRPs as Promising Diagnostic, Prognostic, and Therapeutic Markers in the Clinical Management of Haematological Malignancies
Haematological malignancies represent a mixed group of neoplasms generally divided into leukaemia and lymphoma, depending on their organs of origin. Leukaemia is based on the accumulation of immature cells by mainly affecting blood-forming cells in the bone marrow, and it is classified, according to the blood lineage, in myeloid (acute and chronic) and lymphoid (acute and chronic). Lymphoma is the neoplastic transformation of B and T lymphocytes derived from primary or secondary lymphoid organs and it is classified as non-Hodgkin and Hodgkin lymphoma [30][31].
Table 2. Expression of TRP channels in leukaemia and lymphoma cell line.
| Cancer Type | Cell Line/Model | TRP | Methods | Functions | References |
|---|---|---|---|---|---|
| ALL | Jurkat | TRPV5/V6 | RT-PCR, WB | Early endosome formation | [33][34] |
| T-ALL patient | TRPV1 | RT-PCR | Proliferation Cell death |
[35] | |
| B-ALL patient | TRPC | FISH, RT-PCR | (?) | [36] | |
| B-ALL patient | TRPML2 | RT-PCR | (?) | [37] | |
| Jurkat | TRPV2 | PCR | Deformation stretch-activated current | [38][39] | |
| Jurkat | TRPM2 | qRT-PCR, WB | ROS production | [40] | |
| Jurkat | TRPV1 | RT-PCR | Cell proliferation | [35] | |
| Jurkat | TRPV6 | RT-PCR, WB | Cell migration | [34][41] | |
| Nalm-6 Reh |
TRPC3 | Functional studies | ROS production, Cell death |
[42] | |
| AML | U937 THP1 |
TRPV2 | RT-PCR, WB RT-PCR, WB |
Cell growth, apoptosis, and cell cycle (?) |
[43] |
| THP1 | TRPA1 | Immunofluorescence | Macrophage cytotoxicity | [44][45] | |
| U937 | TRPM2 | RT-PCR, WB | Autophagy | [46][47] | |
| CLL | Jok-1/E1A-E1B | TRPC1 | Flow cytometry | Cytokine production | [48] |
| CML | 32d and 32d-p210 | TRPC1 | WB | Cell proliferation | [49] |
| K562 | TRPM7 | RT-PCR, IF | Erythroid differentiation | [50] | |
| K562 | TRPV2 | RT-PCR, WB | Cell death | [43] | |
| Lymphoma | Daudi B-cell | TRPC6 | WB | Cell proliferation | [51] |
It is now well accepted that alterations of TRP expression and functions are responsible for the impairment of cellular signalling pathways involved in cancer growth, metastasis, and chemoresistance [52]. In particular, dysregulations of TRPC, TRPM, and TRPV members have been mainly correlated with malignant growth and progression. For this reason, in recent years, many efforts have been spent to improve the knowledge about these channels and the ability to target them. Thus, they are considered promising tools to inhibit cancer progression and to ameliorate the diagnosis and overcome chemoresistance in cancer [27][53][54][55][56].
Interesting findings indicate and suggest that TRP channels could also be useful as diagnostic, prognostic, and therapeutic markers in the clinical management of haematological malignancies.
Table 3. Expression and functions of TRP channels in leukaemia and lymphoma patients.
In this regard, integrated methylome, transcriptome, and epigenetic analysis showed that the expression level of many genes is dysregulated in paediatric leukaemia, and among them, the presence of TRPC1, TRPC4, TRPC3, TRPM2, TRPM4, and TRPM8 also stands out. These TRP channels are repressed, and this indicates a reduced potential for cell–microenvironment interactions and apoptotic potential of the ALL cells [36][37]. In accordance with these data, the potential utility to add TRP as diagnostic tools is also confirmed by other studies. It has been found that TRPM4 is significantly overexpressed in diffuse large B-cell lymphoma (DLBCL), compared to normal germinal centre (GC) B cells and, in addition, it is more expressed in activated B-cell-like than in GC DLBCL [61]. Moreover, Hirai et al. demonstrated that TRPM8 positive neoplastic cells are mostly present in post-GC neoplasms but not in pre-GC or in the majority of GC neoplasms, suggesting TRPM8 as a marker to discriminate and diagnose reactive plasmablasts and mature B-neoplasms [60].
Finally, TRPM2 was found to be strongly upregulated in AML samples from patients with normal karyotypes or all AML mutational subgroups with respect to normal hematopoietic stem cells or common myeloid progenitor, suggesting the possibility to differentiate normal from neoplastic cells by using TRPM2 expression levels [47].
The potential prognostic impact of TRP channels in haematological malignancies has been recently highlighted. In DLBCL, the negativity of TRPM4 expression significantly correlated with better overall survival (OS) and progressive-free survival (PFS), compared with TRPM4 strong intensity. TRPM4 positivity was also associated with higher lactate dehydrogenase levels, higher Eastern Cooperative Oncology Group (ECOG) score, and stage III-IV. In addition, TRPM4-positive DLBCL patients treated with R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) protocol displayed worse survival, consistent with its expression [61]. Moreover, a close relationship between TRPM8 and International Prognostic Index (IPI) scores was found in DLBCL patients, indicating that lower TRPM8 expression levels are associated with higher IPI scores [60].
In vivo studies underline the importance of developing new pharmacological approaches based on the targeting of TRP channels. In fact, mice injected with TRPM2-depleted leukaemia cells showed significantly reduced leukaemia, compared to controls, suggesting that these channels play an important role in leukemogenesis [47]. The targeting of TRP channels has been shown to affect leukaemia cells and the tumour microenvironment. It has been recently demonstrated that TRPV4, by acting as a volume receptor, is involved in bone marrow adipocyte remodelling in AML mice, and it is clear that the inhibition of this remodelling increases the survival in the AML mouse model [58].
Moreover, many in vitro studies, performed in patient-derived cells, demonstrated the ability of TRP-targeting therapy to inhibit cell proliferation and improve the effects of traditional chemotherapy in haematological malignancies. For instance, the TRPC3 channel blocker, Pyr3, enhances apoptosis induced by dexamethasone in ALL cells isolated from patients by altering calcium signalling, mitochondrial membrane potential, and ROS production [42]. In addition, the activation of TRPV1, by using the specific agonist RTX, reduced cell proliferation, blocked cell cycle, and increased apoptosis in T cells from ALL patients [35].
Obviously, further studies are necessary in order to develop and use drugs capable of modulating the TRP functions. However, interesting assumptions are gradually emerging. In fact, by molecular imaging methods, the in vivo potential of soricidin-derived peptides in targeting TRPV6-rich tumours has been evaluated [62]. In addition, an apoptosis-inducing TRPV1 nanoagonist containing semiconducting polymer nanoparticles (SPNs) as nanocarriers and CPS as the agonist has been developed to target TRPV1-positive cancer cells [41]. Finally, given that ongoing clinical trials specifically targeting TRPM4 have been approved in patients with stroke [63], it can be expected that, soon, other TRP-based therapeutic strategies may have an adequate safety profile and be applied in the field of cancer, including haematological malignancies.
3. Conclusions
Although TRP channels are found to be important in the carcinogenesis of many tumours so far, little is known about their involvement in leukaemia and lymphoma [32][54][56]. Findings gathered thus far demonstrate that TRPs display an oncogenic activity in haematological malignancies associated with alteration of their molecular expression profile. Given that targeting of TRPs could alter the signalling pathways associated with leukaemia, the evaluation of TRPs as promising biomarkers seems to be of particular interest in the diagnosis, prognosis, and/or treatment of haematological malignancies.
Taken together, these data suggest that attention should be focused on these channels, and increasing efforts should be spent on better characterising their role and function in blood disorders to provide further oncologic targets for upgrading precision medicine.
References
- Nilius, B. TRP channels in disease. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 805–812.
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647.
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323.
- Zheng, J. Molecular Mechanism of TRP Channels. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 3, pp. 221–242.
- Duan, J.; Li, Z.; Li, J.; Hulse, R.E.; Santa-Cruz, A.; Valinsky, W.C.; Abiria, S.A.; Krapivinsky, G.; Zhang, J.; Clapham, D.E. Structure of the mammalian TRPM7, a magnesium channel required during embryonic development. Proc. Natl. Acad. Sci. USA 2018, 115, E8201–E8210.
- Ferrandiz-Huertas, C.; Mathivanan, S.; Wolf, C.J.; Devesa, I.; Ferrer-Montiel, A. Trafficking of thermo TRP channels. Membranes 2014, 4, 525–564.
- Méndez-Reséndiz, K.A.; Enciso-Pablo, Ó.; González-Ramírez, R.; Juárez-Contreras, R.; Rosenbaum, T.; Morales-Lázaro, S.L. Steroids and TRP Channels: A close relationship. Int. J. Mol. Sci. 2020, 21, 3819.
- Nagatomo, K.; Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 2008, 105, 17373–17378.
- Talavera, K.; Gees, M.; Karashima, Y.; Meseguer, V.M.; Vanoirbeek, J.A.J.; Damann, N.; Everaerts, W.; Benoit, M.; Janssens, A.; Vennekens, R.; et al. Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 2009, 12, 1293–1299.
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857.
- Rubaiy, H.N. Treasure troves of pharmacological tools to study transient receptor potential canonical 1/4/5 channels. Br. J. Pharmacol. 2019, 176, 832–846.
- Venkatachalam, K.; Zheng, F.; Gill, D.L. Regulation of Canonical Transient Receptor Potential (TRPC) Channel Function by Diacylglycerol and Protein Kinase, C.J. Biol. Chem. 2003, 278, 29031–29040.
- Leuner, K.; Kazanski, V.; Muller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin—A key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 2007, 21, 4101–4111.
- Leuner, K.; Heiser, J.H.; Derksen, S.; Mladenov, M.I.; Fehske, C.J.; Schubert, R.; Gollasch, M.; Schneider, G.; Harteneck, C.; Chatterjee, S.S.; et al. Simple 2,4-Diacylphloroglucinols as classic transient receptor Potential-6 activators—Identification of a novel pharmacophore. Mol. Pharmacol. 2010, 77, 368–377.
- García-Ávila, M.; Islas, L.D. What is new about mild temperature sensing? A review of recent findings. Temperature 2019, 6, 132–141.
- Kraft, R.; Grimm, C.; Frenzel, H.; Harteneck, C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br. J. Pharmacol. 2006, 148, 264–273.
- Hill, K.; McNulty, S.; Randall, A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn. Schmiedebergs. Arch. Pharmacol. 2004, 370, 227–237.
- Hill, K.; Benham, C.; McNulty, S.; Randall, A. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 2004, 47, 450–460.
- Malysz, J.; Maxwell, S.; Yarotskyy, V.; Petkov, G.V. Compound-dependent effects of TRPM4 Channel Modulators on Guinea Pig detrusor smooth muscle excitability and contractility. FASEB J. 2019, 33, 837.4.
- Takezawa, R.; Cheng, H.; Beck, A.; Ishikawa, J.; Launay, P.; Kubota, H.; Kinet, J.-P.; Fleig, A.; Yamada, T.; Penner, R. A Pyrazole derivative potently inhibits lymphocyte Ca2+ Influx and cytokine production by facilitating transient Receptor Potential Melastatin 4 Channel Activity. Mol. Pharmacol. 2006, 69, 1413–1420.
- Wong, R.; Turlova, E.; Feng, Z.-P.; Rutka, J.T.; Sun, H.-S. Activation of TRPM7 by naltriben enhances migration and invasion of glioblastoma cells. Oncotarget 2017, 8, 11239–11248.
- Saldanha, S.; Grimm, C.; Mercer, B.; Choi, J.; Allais, C.; Roush, W.; Heller, S.; Hodder, P. Agonists of Transient Receptor Potential Channels 3 and 2 (TRPML3 & TRPML2). Probe Rep. NIH Mol. Libr. Progr. Internet 2009, 2, 1–21.
- Plesch, E.; Chen, C.-C.; Butz, E.; Scotto Rosato, A.; Krogsaeter, E.K.; Yinan, H.; Bartel, K.; Keller, M.; Robaa, D.; Teupser, D.; et al. Selective agonist of TRPML2 reveals direct role in chemokine release from innate immune cells. Elife 2018, 7, e39720.
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507.
- Chow, J.; Norng, M.; Zhang, J.; Chai, J. TRPV6 mediates capsaicin-induced apoptosis in gastric cancer cells--Mechanisms behind a possible new “hot” cancer treatment. Biochim. Biophys. Acta 2007, 1773, 565–576.
- Dong, X.P.; Wang, X.; Xu, H. TRP channels of intracellular membranes. J. Neurochem. 2010, 113, 313–328.
- Caterina, M.J.; Pang, Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals 2016, 9, 77.
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17.
- Hempel, N.; Trebak, M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017, 63, 70–96.
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405.
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423.
- Morelli, M.B.; Liberati, S.; Amantini, C.; Nabiss, M.; Santoni, M.; Farfariello, V.; Santoni, G. Expression and function of the transient receptor potential ion channel family in the hematologic malignancies. Curr. Mol. Pharmacol. 2013, 6, 137–148.
- Tomilin, V.N.; Cherezova, A.L.; Negulyaev, Y.A.; Semenova, S.B. TRPV5/V6 Channels Mediate Ca 2+ Influx in Jurkat T Cells under the control of extracellular pH. J. Cell. Biochem. 2016, 117, 197–206.
- Vassilieva, I.O.; Tomilin, V.N.; Marakhova, I.I.; Shatrova, A.N.; Negulyaev, Y.A.; Semenova, S.B. Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in human blood lymphocytes and Jurkat leukemia T cells. J. Membr. Biol. 2013, 246, 131–140.
- Punzo, F.; Manzo, I.; Tortora, C.; Pota, E.; D’Angelo, V.; Bellini, G.; Di Paola, A.; Verace, F.; Casale, F.; Rossi, F. Effects of CB2 and TRPV1 receptors’ stimulation in pediatric acute T-lymphoblastic leukemia. Oncotarget 2018, 9, 21244–21258.
- Chatterton, Z.; Morenos, L.; Mechinaud, F.; Ashley, D.M.; Craig, J.M.; Sexton-Oates, A.; Halemba, M.S.; Parkinson-Bates, M.; Ng, J.; Morrison, D.; et al. Epigenetic deregulation in pediatric acute lymphoblastic leukemia. Epigenetics 2014, 9, 459–467.
- Almamun, M.; Levinson, B.T.; van Swaay, A.C.; Johnson, N.T.; McKay, S.D.; Arthur, G.L.; Davis, J.W.; Taylor, K.H. Integrated methylome and transcriptome analysis reveals novel regulatory elements in pediatric acute lymphoblastic leukemia. Epigenetics 2015, 10, 882–890.
- Pottosin, I.; Delgado-Enciso, I.; Bonales-Alatorre, E.; Nieto-Pescador, M.G.; Moreno-Galindo, E.G.; Dobrovinskaya, O. Mechanosensitive Ca(2)(+)-permeable channels in human leukemic cells: Pharmacological and molecular evidence for TRPV2. Biochim. Biophys. Acta 2015, 1848, 51–59.
- Wenning, A.S.; Neblung, K.; Strauß, B.; Wolfs, M.J.; Sappok, A.; Hoth, M.; Schwarz, E.C. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 412–423.
- Klumpp, D.; Misovic, M.; Szteyn, K.; Shumilina, E.; Rudner, J.; Huber, S.M. Targeting TRPM2 channels impairs radiation-induced cell cycle arrest and fosters cell death of T cell leukemia cells in a Bcl-2-dependent manner. Oxid. Med. Cell. Longev. 2016, 2016.
- Bobkov, D.; Yudintceva, N.; Lomert, E.; Shatrova, A.; Kever, L.; Semenova, S. Lipid raft integrity is required for human leukemia Jurkat T-cell migratory activity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 2–11.
- Abdoul-Azize, S.; Buquet, C.; Vannier, J.P.; Dubus, I. Pyr3, a TRPC3 channel blocker, potentiates dexamethasone sensitivity and apoptosis in acute lymphoblastic leukemia cells by disturbing Ca 2+ signaling, mitochondrial membrane potential changes and reactive oxygen species production. Eur. J. Pharmacol. 2016, 784, 90–98.
- Siveen, K.S.; Prabhu, K.S.; Parray, A.S.; Merhi, M.; Arredouani, A.; Chikri, M.; Uddin, S.; Dermime, S.; Mohammad, R.M.; Steinhoff, M.; et al. Evaluation of cationic channel TRPV2 as a novel biomarker and therapeutic target in Leukemia-Implications concerning the resolution of pulmonary inflammation. Sci. Rep. 2019, 9, 1554.
- Tian, C.; Huang, R.; Tang, F.; Lin, Z.; Cheng, N.; Han, X.; Li, S.; Zhou, P.; Deng, S.; Huang, H.; et al. Transient Receptor Potential Ankyrin 1 Contributes to Lysophosphatidylcholine-Induced Intracellular Calcium Regulation and THP-1-Derived Macrophage Activation. J. Membr. Biol. 2020, 253, 43–55.
- Tian, C.; Han, X.; He, L.; Tang, F.; Huang, R.; Lin, Z.; Li, S.; Deng, S.; Xu, J.; Huang, H.; et al. Transient receptor potential ankyrin 1 contributes to the ATP-elicited oxidative stress and inflammation in THP-1-derived macrophage. Mol. Cell. Biochem. 2020, 473, 179–192.
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17.
- Chen, S.; Bao, L.; Keefer, K.; Shanmughapriya, S.; Chen, L.; Lee, J.; Wang, J.; Zhang, X.-Q.; Hirschler-Laszkiewicz, I.; Merali, S.; et al. Transient receptor potential ion channel TRPM2 promotes AML proliferation and survival through modulation of mitochondrial function, ROS, and autophagy. Cell Death Dis. 2020, 11, 247.
- Garaud, S.; Taher, T.E.; Debant, M.; Burgos, M.; Melayah, S.; Berthou, C.; Parikh, K.; Pers, J.O.; Luque-Paz, D.; Chiocchia, G.; et al. CD5 expression promotes IL-10 production through activation of the MAPK/Erk pathway and upregulation of TRPC1 channels in B lymphocytes. Cell. Mol. Immunol. 2018, 15, 158–170.
- Cabanas, H.; Harnois, T.; Magaud, C.; Cousin, L.; Constantin, B.; Bourmeyster, N.; Déliot, N. Deregulation of calcium homeostasis in Bcr-Abl-dependent chronic myeloid leukemia. Oncotarget 2018, 9, 26309–26327.
- Takahashi, K.; Umebayashi, C.; Numata, T.; Honda, A.; Ichikawa, J.; Hu, Y.; Yamaura, K.; Inoue, R. TRPM7-mediated spontaneous Ca2+ entry regulates the proliferation and differentiation of human leukemia cell line K562. Physiol. Rep. 2018, 6, 1–15.
- Song, X.; Liu, B.; Lu, X.; Yang, L.; Zhai, Y.; Eaton, A.F.; Thai, T.L.; Eaton, D.C.; Ma, H.; Shen, B. Biochimica et Biophysica Acta Lovastatin inhibits human B lymphoma cell proliferation by reducing intracellular ROS and TRPC6 expression. BBA Mol. Cell Res. 2014, 1843, 894–901.
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin. Immunopathol. 2016, 38, 357–369.
- Santoni, G.; Farfariello, V. TRP channels and cancer: New targets for diagnosis and chemotherapy. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 54–67.
- Liberati, S.; Morelli, M.B.; Nabissi, M.; Santoni, M.; Santoni, G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr. Top. Med. Chem. 2013, 13, 344–366.
- Smani, T.; Shapovalov, G.; Skryma, R.; Prevarskaya, N.; Rosado, J.A. Functional and physiopathological implications of TRP channels. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1772–1782.
- Santoni, G.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Amantini, C. Calcium signaling and the regulation of chemosensitivity in cancer cells: Role of the transient receptor potential channels. Adv. Exp. Med. Biol. 2020, 1131, 505–517.
- Gil-Kulik, P.; Dudzińska, E.; Radzikowska-Büchner, E.; Wawer, J.; Jojczuk, M.; Nogalski, A.; Wawer, G.A.; Feldo, M.; Kocki, W.; Cioch, M.; et al. Different regulation of PARP1, PARP2, PARP3 and TRPM2 genes expression in acute myeloid leukemia cells. BMC Cancer 2020, 20, 1–9.
- Yang, S.; Lu, W.; Zhao, C.; Zhai, Y.; Wei, Y.; Liu, J.; Yu, Y.; Li, Z.; Shi, J. Leukemia cells remodel marrow adipocytes via TRPV4-dependent lipolysis. Haematologica 2019.
- Debant, M.; Burgos, M.; Hemon, P.; Buscaglia, P.; Fali, T.; Melayah, S.; Le Goux, N.; Vandier, C.; Potier-Cartereau, M.; Pers, J.O.; et al. STIM1 at the plasma membrane as a new target in progressive chronic lymphocytic leukemia. J. Immunother. Cancer 2019, 7, 1–13.
- Hirai, A.; Aung, N.Y.; Ohe, R.; Nishida, A.; Kato, T.; Meng, H.; Ishizawa, K.; Fujii, J.; Yamakawa, M. Expression of TRPM8 in human reactive lymphoid tissues and mature B-cell neoplasms. Oncol. Lett. 2018, 16, 5930–5938.
- Loo, S.K.; Ch’ng, E.S.; Md Salleh, M.S.; Banham, A.H.; Pedersen, L.M.; Moller, M.B.; Green, T.M.; Wong, K.K. TRPM4 expression is associated with activated B cell subtype and poor survival in diffuse large B cell lymphoma. Histopathology 2017, 71, 98–111.
- Bowen, C.V.; DeBay, D.; Ewart, H.S.; Gallant, P.; Gormley, S.; Ilenchuk, T.T.; Iqbal, U.; Lutes, T.; Martina, M.; Mealing, G.; et al. In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS ONE 2013, 8, e58866.
- Chen, B.; Gao, Y.; Wei, S.; Low, S.W.; Ng, G.; Yu, D.; Tu, T.M.; Soong, T.W.; Nilius, B.; Liao, P. TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Pflügers Arch. Eur. J. Physiol. 2019, 471, 1455–1466.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
23 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




