Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafael Nóbrega | + 3393 word(s) | 3393 | 2021-06-09 04:27:18 | | | |
| 2 | Vivi Li | Meta information modification | 3393 | 2021-06-10 03:52:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nóbrega, R. Spermatogonial Stem Cells in Fish. Encyclopedia. Available online: https://encyclopedia.pub/entry/10702 (accessed on 07 February 2026).
Nóbrega R. Spermatogonial Stem Cells in Fish. Encyclopedia. Available at: https://encyclopedia.pub/entry/10702. Accessed February 07, 2026.
Nóbrega, Rafael. "Spermatogonial Stem Cells in Fish" Encyclopedia, https://encyclopedia.pub/entry/10702 (accessed February 07, 2026).
Nóbrega, R. (2021, June 09). Spermatogonial Stem Cells in Fish. In Encyclopedia. https://encyclopedia.pub/entry/10702
Nóbrega, Rafael. "Spermatogonial Stem Cells in Fish." Encyclopedia. Web. 09 June, 2021.
Copy Citation
Spermatogenesis is a continuous and dynamic developmental process, in which a single diploid spermatogonial stem cell (SSC) proliferates and differentiates to form a mature spermatozoon. Herein, we summarize the accumulated knowledge of SSCs and their distribution in the testes of teleosts.
spermatogonial stem cell (SSC)
fish
spermatogenesis
florescence-activated cell sorting (FACS)
magnetic-activated cell sorting (MACS)
germ cell culture
1. Spermatogenesis—an Overview
Spermatogenesis is a continuous and dynamic developmental process which can be divided into three phases: the mitotic, or spermatogonial, phase with the generation of spermatogonia; the meiotic phase with primary and secondary spermatocytes; and the spermiogenic phase with the haploid spermatids emerging from meiosis and differentiating into motile, flagellated, haploid spermatozoa.
In the spermatogonial phase, the primary increase in germ cell numbers occurs during successive rounds of mitotic duplication of the spermatogonia. The number of spermatogonial generations, and hence the number of mitotic divisions before differentiation into spermatocytes, varies among, but not within, species. There can be as few as two (humans) and as many as 14 (guppy) generations, but, most commonly, five to eight generations are reported [1]. In this phase, in all invertebrates and vertebrates, at the end of mitosis, incomplete cytokinesis occurs, and the two newly-generated spermatogonia remain connected by a cytoplasmic bridge, instead of forming individual cells (Figure 1A,B). However, the cytoplasmic bridge is not present in the descendants of an SSC that enters a self-renewal pathway in which two single, isolated daughter cells are generated. Therefore, cytoplasmic bridges are considered a sign of SSC differentiation and are present during all subsequent germ cell divisions (Figure 1B). All differentiated descendants of an SSC form clones connected by cytoplasmic bridges through which their developmental steps are synchronized (Figure 1A). These bridges are cleaved when spermatogenesis is complete, and germ cells leave the germinal epithelium as spermatozoa [2].
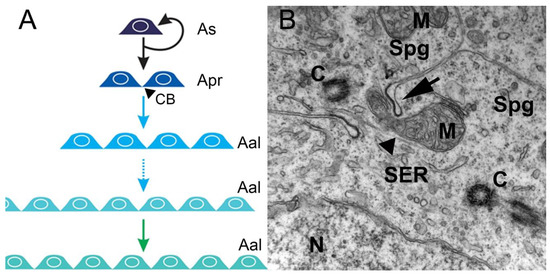
Figure 1. Mitotic/spermatogonial phase. (A) Mitosis produces spermatogonium clones. In rodents, type A single spermatogonia (As) harbor the spermatogonial stem cell population, which can either self-renew or generate two interconnected cells named type A-paired spermatogonia (Apr). The A-paired spermatogonia are interconnected by cytoplasmic bridge (CB) as a consequence of incomplete cytokinesis during cell division. Amplifying divisions beyond the A-paired also do not complete cytokinesis and continue to generate longer syncytial chains, termed A-aligned spermatogonia (Aal). (B). An electron micrograph showing a cytoplasmic bridge (arrow) connecting daughter cells resulting from spermatogonium division. The mitochondria (M), nucleus (N), smooth endoplasmic reticulum (SER), centrioles (C), and microtubules (arrowhead) are depicted in the cytoplasms of two interconnected spermatogonia (Spg) (illustration and data: Nóbrega—unpublished observations).
During the meiotic phase, the spermatogonia differentiate into spermatocytes that go through two meiotic divisions characterized by reshuffling of the parental genetic material during the first division and the reduction to a haploid genome at the second division [1].
In the spermiogenic phase, the haploid spermatids emerging from meiosis differentiate into flagellated spermatozoa without further proliferation. The morphological changes in germ cells occurring during spermiogenesis involve reduction in cytoplasmic volume and organelles, maximum DNA condensation, and differentiation of the flagellum, and are similar among species. However, the final spermatozoon morphology can differ and sometimes provides taxonomic discrimination [1].
Following the general vertebrate scheme, the testes of fish are composed of the interstitial or intertubular compartment, and the germinal or tubular compartment, separated by a basement membrane [3]. The interstitial compartment contains the steroid-producing Leydig cells, blood/lymph vessels, macrophages, granulocytes, and connective tissue elements [4]. The peritubular myoid cells form a single layer of flattened cells surrounding the seminiferous tubules [5]. These cells are contractile and involved in the transport of spermatozoa and testicular fluid in the tubule [5]. The germinal compartment is composed of germ cells at various stages of development and their associated somatic Sertoli cells, which together form the germinal epithelium [3]. In the epithelium, germ cell survival and development depend on constant close contact with Sertoli cells [6]. Although many features are conserved in vertebrate spermatogenesis, the Sertoli/germ cell association differs among vertebrates. While anamniotes (fishes and amphibians) exhibit so-called cystic spermatogenesis, the amniotes (reptiles, birds, and mammals) present non-cystic spermatogenesis [3], in which the seminiferous epithelium can be divided into separate stages according to the cell associations observed in each tubular cross-section [7].
The germinal epithelium of amniote adult testes is composed of a fixed number of “immortal” Sertoli cells that support successive waves of spermatogenesis [6][7]. During these waves, a given Sertoli cell simultaneously supports several germ cell developmental stages (i.e., cells belonging to different germ cell clones). The Sertoli cell base may contact spermatogonia, with lateral segments contacting spermatocytes and early spermatids, and adluminal segments contacting late spermatids. In this type of spermatogenesis, Sertoli cells have been shown to be terminally differentiated in the testes, ceasing division during the early pre-pubertal phase, from approximately 10 and 20 days after birth in mice and rats, respectively [6][8]. However, recent studies have demonstrated that Sertoli cells from the transition region between the seminiferous tubules and the rete testis of adult testes remain undifferentiated for a longer period and are able to proliferate, although at a lower rate [8].
In anamniote vertebrates, the germinal epithelium is composed of spermatogenic cysts. The cyst as a morpho-functional unit is formed when a group of Sertoli cells envelope a single SSC [9]. As spermatogonia divide, the derived cells remain interconnected by cytoplasmic bridges [1][3][6]. Thus, the anaminiote Sertoli cell supports a single germ cell clone, while in amniote testes, depending on species, at least five germ cell clones at different stages of development are supported by a single Sertoli cell [1][3][6]. Sertoli cells from anaminiote testes are able to continuously proliferate, even after the onset of puberty [10].
The structural differences in the Sertoli/germ cell relationship of anamniotes and amniotes result in a less complex situation in fish than exists in mammals. Cystic spermatogenesis proceeds in a sequential manner, while, in non-cystic spermatogenesis, several processes occur simultaneously [6]. In vertebrates, the endocrine system has evolved as the master control system of spermatogenesis, and the somatic Sertoli, Leydig, and peritubular myoid cells became the primary targets for the major reproductive hormones, acting as paracrine relay stations for these signals in the testes [11][12].
2. Spermatogonial Stem Cell Niche
Spermatogenesis relies on the activity of SSCs, which are capable of self-renewal to produce more stem cells or differentiation into daughter cells dedicated to spermatogenesis [13][14][15][16][17]. The balance between SSC self-renewal and differentiation is the basis of maintaining the homeostasis of spermatogenesis. If one process takes precedence over the other, testicular cancer (in case of self-renewal) or a depletion of spermatogenesis (in differentiation) is the outcome (Figure 2).
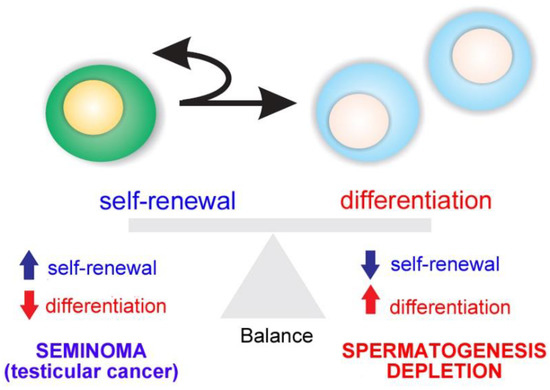
Figure 2. Balance between spermatogonial stem cell (SSC) self-renewal and differentiation. An imbalance results in testicular cancer or depletion of spermatogenesis.
The simultaneous process occurs in vertebrates showing continuous spermatogenesis, while, in seasonal breeding species, a switch from self-renewal to differentiation is observed as gonads begin to mature [1].
Spermatogonial stem cells are maintained in a specialized microenvironment in the testes known as the testicular niche (Figure 3) [14][15][16][18][19][20][21]. The niche provides growth factors and cell-to-cell interactions that regulate SSC activity in the testes: cell-cycle quiescence, maintenance of the undifferentiated state, proliferation via self-renewal, and apoptosis (Figure 3) [14][15][16].
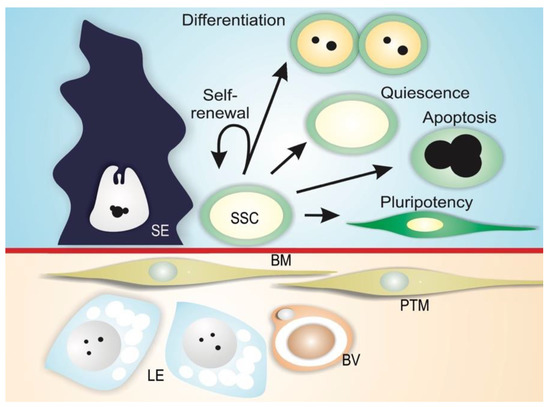
Figure 3. Mammalian SSC niche. Spermatogonial stem cells (SSC) reside along the basement membrane (BM) in proximity to interstitial Leydig cells (LE) and blood vessels (BV), and are in contact with Sertoli cells (SE). In this microenvironment, physical and paracrine interactions regulate SSC self-renewal, differentiation, quiescence, apoptosis, and the ability to transform into different cell types (pluripotency). Peritubular myoid cells (PTM) are also depicted in the figure.
The niche can be defined as a microenvironment that maintains the undifferentiated state of a stem cell by preventing its differentiation, and is usually composed of (1) supporting cells; (2) stem cells; and (3) the surrounding extracellular matrix (Figure 3) [22][23][24]. In mammals, SSCs lie on the basement membrane of the seminiferous epithelium and are in contact with Sertoli cells, which control the fate of the SSCs via physical and paracrine interactions [16][25][26][27]. In addition to Sertoli cells, peritubular myoid cells and Leydig cells may contribute soluble growth factors to the niche environment [28][29][30]. Mammalian SSCs are preferentially located in those areas of the seminiferous tubules near the interstitial tissue where Leydig cells and blood vessels reside (Figure 3) [29][31][32][33][34]. Recent studies have shown that type A undifferentiated spermatogonia are uniformly distributed on the basement membrane of seminiferous mouse epithelium [35]. It has been demonstrated that SSC self-renewal and proliferation are intensified in areas of high fibroblast growth factor (FGF), which corresponds to vasculature-proximal and interstitium-proximal regions, and SSCs must be exposed to a sufficiently high level of FGF in order to maintain the self-renewal state [36].
In anamniote species, SSCs and their niche remain poorly investigated. In fish and other anamniotes, SSCs are single cells, not lying directly on the basement membrane, but completely enclosed by Sertoli cell cytoplasmic extensions [1]. Similarly to mammals, fish have SSCs that are considered type A undifferentiated spermatogonia (Aund) [13][1]. There is evidence for two sub-types of single, undifferentiated spermatogonia, type Aund* and type Aund, in several fish species (Figure 4A–C), including ancient species, such as sterlet Acipenser ruthenus (Figure 4D–G) [1][18][37]. The Aund* spermatogonia exhibit a large nucleus with little heterochromatin; a high volume of the cytoplasm; a convoluted nuclear envelope; evident nucleoli; and particularly relevantly, darkly staining material near invaginations of the convoluted nuclear envelope [1][37]. These patches are known as “nuage,” material composed of ribonucleic acid (RNA) and RNA-processing proteins [1]. The Aund spermatogonia show a smooth nuclear envelope, darker chromatin with abundant patches of heterochromatin distributed in the nucleus, and less nuage [1][37].
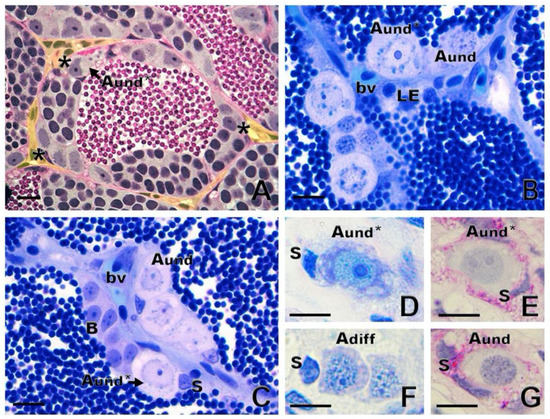
Figure 4. Spermatogonial niche and type A undifferentiated (Aund) spermatogonia. (A) Asterisks show the most undifferentiated type A spermatogonia (Aund*), preferentially located near the interstitial compartment (delimited in yellow) in zebrafish Danio rerio. (B,C) In common carp Cyprinus carpio, both Aund* and Aund are located near the interstitium, close to Leydig cells (LE) and blood vessels (bv). Sertoli cell (S) and type B spermatogonia are shown. (D–G): Generations of sterlet Acipenser ruthenus spermatogonia: Aund* and Aund and type A differentiated (Adiff) spermatogonia. Staining: periodic acid–Schiff (A,E,G) and toluidine blue (B,C,D,F). Scale bar: 10 µm.
These cells also exhibit differences in the cell cycle, as shown by bromodeoxyuridine 5-bromo-2’-deoxyuridine (BrdU), a marker of S-phase in pulse-chase experiments [18]. Nóbrega and collaborators [18], using BrdU pulse-chase, reported that BrdU was rapidly diluted in type Aund spermatogonia, while the percentage of BrdU-positive Aund* spermatogonia remained constant. The authors [18] suggested that type Aund constitute an “active” population with rapid proliferation and differentiation, as indicated by the more rapid loss of BrdU; Aund* are the “reserve” population with slow self-renewal proliferation, as indicated by relatively stable BrdU labeling. Two types of single A spermatogonia in humans display similar characteristics: a “pale” type acting as reserve, and a “dark,” active type [38][39]. In medaka Oryzias latipes, Nakamura and collaborators [40], using a BrdU pulse-chase experiment, also revealed distinct rapid and slow-dividing populations of oogonial stem cells.
With respect to whether spermatogonia display a preferential distribution within the fish testes, studies of zebrafish [18] and Astyanax altiparane [41] have shown that both Aund* and Aund spermatogonia are located near the interstitial compartment, in contact with androgen-producing Leydig cells and blood vessels (Figure 4A–C). These observations suggest that the androgens; growth factors; and vascular supplies of oxygen, nutrients, and hormones may play essential roles in SSC maintenance and self-renewal vs. differentiation in the fish testis niche [1][12].
Functional assays have been reported that consist of transplanting SSCs from a donor into a recipient testis in which endogenous spermatogenesis has been blocked [42]. Depending on self-renewal and differentiation capacities, transplanted spermatogonia are able to colonize the recipient testis and differentiate into functional gametes [42]. Studies have demonstrated donor-derived spermatogenesis following type Aund spermatogonia transplantation into recipient testes in several species of fish [18][37][43][44][45]. Type A undifferentiated spermatogonia have also been shown to exhibit sexual plasticity and the ability to de-differentiate and differentiate into oocytes when transplanted into zebrafish ovaries [18]. In addition, Aund spermatogonia transplanted into sexually undifferentiated larvae were able to differentiate into functional spermatozoa or eggs, depending on the genetic sex of the recipient [46][47]. Taken together, these findings provide evidence that SSCs represent a subset of type Aund spermatogonia.
Although available information on fish SSCs has expanded in recent decades, markers for SSCs have been identified in only a few species [48], presenting limitations to detecting and isolating SSCs. Similar to the situation in mammals, in the past decade, spermatogonium transplant was the only means of assessing the “stemness” of putative SSCs in fish [20][44][49][50][51].
There is evidence that promyelocytic leukemia zinc finger protein (PLZF), a transcription repressor essential for the maintenance of mammalian SSCs [52], can be a marker of SSCs in fish, as demonstrated in the rohu Labeo rohita [53], zebrafish [54][55], dogfish Scyliorhinus canicula [56], rainbow trout Oncorhynchus mykiss [57], and several species of catfish [58][59][60]. In the neotropical catfish Rhamdia quelen, in situ hybridization showed that plzf is strongly expressed in type Aund spermatogonia, but was also detected in type Adiff spermatogonia, although at less intensity [60].
Glial cell-derived neurotrophic factor (GDNF) is a Sertoli cell growth factor involved in mammalian SSC maintenance [61]. The GDNF-binding receptor GDNF family receptor alpha1 (GFRα1) attaches to the membrane of SSCs, and is considered a SSC marker in several mammalian species [61][62][63][64]. Recently, GFRα1 has been detected in type Aund of Nile tilapia Oreochromis niloticus [65], rainbow trout [66], dogfish [56], and common carp (Figure 5A). In rainbow trout, gdnf is expressed in germ cells from spermatogonia to spermatocytes but not in Sertoli cells, indicating that it is not secreted as an autocrine SSC niche factor in rainbow trout testes, unlike in mammals [66].
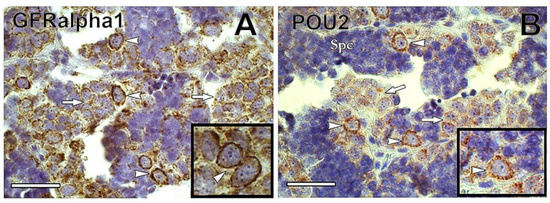
Figure 5. (A) Immunoreactivity of GFRα1 was found preferentially in type A undifferentiated spermatogonia (arrowheads) of common carp. In contrast, GFRα1 immunoreactivity decreased in differentiated spermatogonia (arrows). The inset shows GFRα1-positive undifferentiated spermatogonia (arrowheads). (B) POU2 was detected in common carp type A undifferentiated spermatogonia (arrowheads), whereas its immunoreactivity decreased in differentiated spermatogonia (arrows). Inset shows POU2-positive undifferentiated spermatogonia. Spc = spermatocyte. Scale bar: 25 µm.
Transcription factors NANOG and Pou5f3 (POU family/Oct4) are expressed in SSCs of mammals, and in some fish, including medaka, zebrafish, Nile tilapia, and rainbow trout [57][65][67][68][69]. Both NANOG and Pou5f3 play important roles in the maintenance and self-renewal of undifferentiated and pluripotent cells [70][71]. In medaka gametes, nanog is expressed only in spermatogonia, being absent in somatic cells of ovary and testis [69]. In common carp, POU2 was detected in Aund spermatogonia, decreasing in expression as spermatogonia differentiated (Nobrega et al.—unpublished observations) (Figure 5B).
Recently, an approach to establishing markers for SSC in fish has been developed [72]. The method consisted of generating monoclonal antibodies (mAb) to cell-surface molecules of rainbow trout type Aund spermatogonia by inoculating enriched live Aund spermatogonia into mice and screening with a combination of cell enzyme-linked immunosorbent assay, live-cell staining, and flow cytometry (FCM) [72]. Among the obtained antibodies, two (numbers 80 and 95) were capable of specifically labelling Aund spermatogonia of rainbow trout and zebrafish [72], while other antibodies (numbers 172 and 189) showed strong signals for type A spermatogonia and the oogonia of several species of salmonid [73]. By using these antibodies with fluorescence-activated cell sorting (FACS) [72] or magnetic-activated cell sorting (MACS) [74], it was possible to enrich Aund spermatogonia and increase transplant success rate in selected teleosts. This method presents the potential to identify molecular markers of SSCs in fish, and the potential to isolate and enrich SSCs for downstream studies, such as single-cell RNA-seq or in vitro experiments. Table 1 presents the major SSC markers currently reported in fish.
Table 1. Spermatogonial stem cell markers in fish.
| Marker | Specification | Species | Reference |
|---|---|---|---|
| SGSA-1 | Spermatogonia specific-antigen-1 | Japanese eel | [75] |
| Notch1 | Notch homolog protein 1 | Rainbow trout | [76] |
| Pou5/2 (Oct-4) | POU domain, class 5/2 | Medaka Common carp |
[68] Nobrega et al. unpublished observations |
| Ly75 (CD205) | Lymphocyte antigen 75 | Rainbow trout Pacific bluefin tuna |
[77] [78] |
| PLZF | Promyelocytic leukemia zinc finger | Zebrafish Carpa rohu Dogfish Rainbow trout Catfish (several species) |
[54] [53] [56] [57] [58][59][60] |
| GFRα1 | GDNF-family receptor α1 | Nile tilapia Dogfish Rainbow trout Common carp |
[65] [56] [66] Nobrega et al. unpublished observations |
| NANOG | NANOG homeobox | Medaka Nile tilapia |
[69] [65] |
| NANOS2 | NANOS homolog 2 | Medaka Nile tilapia Rainbow trout |
[79] [65] [57] |
| NANOS3 | NANOS homolog 3 | Rainbow trout | [57] |
| Antibodies numbers 80 and 95 | Not identified | Rainbow trout Also applied in Zebrafish Salmonids |
[72] [72] [74] |
3. Endocrine and Paracrine Regulation
In vertebrates, pituitary gonadotropin follicle stimulating hormone (FSH) and luteinizing hormone (LH) control testicular development, and function by regulating the activity of local signaling systems involving sex steroids and growth factors [80][81], small RNAs [82], and epigenetic switches [83].
In rodents, FSH can modulate the production of Sertoli cell growth factors that are relevant for SSC self-renewal or differentiation [34]. Among the growth factors, the GDNF secreted by Sertoli cells plays an important role in SSC self-renewal [61], while activin A and bone morphogenetic protein 4 (BMP4), also produced by Sertoli cells, promote differentiation [84]. Growth factors produced by Leydig and peritubular myoid cells can also modulate SSC self-renewal or differentiation [28]. For example, colony-stimulating factor 1 secreted by Leydig and some peritubular myoid cells [28] stimulates SSC self-renewal.
The gonadotropic hormones FSH and LH are important for testis development and spermatogenesis in fish [1]. Despite their similar roles in vertebrates, evolution has taken a different path for the gonadotropic hormones and their biological activities in teleost fish when compared to the other vertebrates. Leydig cells express not only the receptor for LH, typically seen in all vertebrates, but also the receptor for FSH [85][86][87][88]. Therefore, FSH can regulate Leydig cell functions, including stimulation of androgen [85][86][87] and production of growth factors acting on spermatogonial self-renewal and differentiation, such as Wnt5a [89] and insulin-like peptide 3 (Insl3) [90], respectively (Figure 6). Studies have reported elevated levels of circulating androgens and FSH coinciding with active spermatogonial proliferation in male Chinook salmon Oncorhynchus tshawytscha, and FSH stimulates spermatogonium proliferation in juvenile Japanese eel Anguilla japonica [85] as well as androgen production in several fish species [85][86][87][91]. Research in immature Japanese eels has shown FSH-induced spermatogenesis to be blocked by trilostane, a steroid hormone synthesis inhibitor, suggesting that FSH effects were mediated by androgens [85]. On the other hand, studies in zebrafish have demonstrated the impact of FSH on germ cell development independent of androgens [11]. This is supported by evidence that hundreds of testicular transcripts respond to FSH but not to sex steroids in the testes of zebrafish [92] and rainbow trout [93]. Most of these genes belong to cellular pathways known to regulate cell proliferation and differentiation, such as insulin-like growth factor (Igf3), Insl3, transforming-growth factor members (Tgf-β), Wnt, Notch, and Hedgehog signaling [92]. Since Sertoli cells express FSH receptor [85][86][87], it is likely that Sertoli cells act as a paracrine relay station for FSH signal in the testes [6].
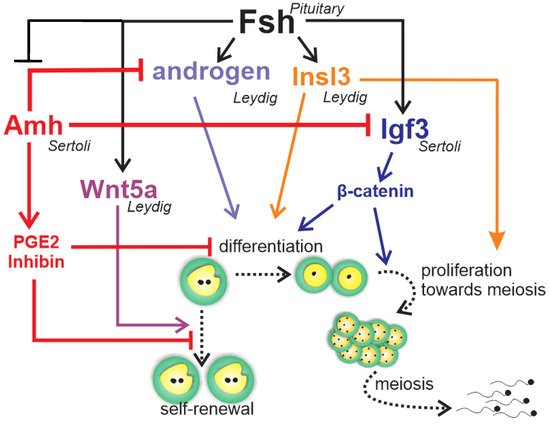
Figure 6. Schematic representation to summarize the regulation of zebrafish spermatogonial phase. Fsh exerts a central role in the zebrafish’s spermatogonial phase by triggering and balancing steroid and growth factor production in testicular somatic cells (Leydig and Sertoli cells). In Leydig cells, Fsh stimulates the production of androgens and Insl3, which are both involved in the spermatogonial differentiation and proliferation. Moreover, Fsh promotes spermatogonial self-renewal by increasing Wnt5a release by Leydig cells. In Sertoli cells, Fsh increases Igf3, which in turn promotes differentiation and proliferation by activating β-catenin signaling in the germ cells. Fsh also down-regulates Amh, a member of the TGF-β (transforming growth factor-beta) superfamily produced by Sertoli cells. Amh, through PGE2 or inhibin, exerts an inhibitory role on spermatogonial self-renewal and germ cell differentiation in the zebrafish testes. To sustain its inhibitory role, Amh also decreases androgen and Igf3 production in Leydig and Sertoli cell, respectively.
Most of the accumulated knowledge regarding the role of growth factors in spermatogonial self-renewal vs. differentiation was derived from zebrafish studies (Figure 6) [11][12][86][92][94][95]. The Tgf-β Amh, expressed in Sertoli cells [94][95][96], has been shown to exert a role in adult teleost gonad development in both males and females, particularly at germ cell early stages [97]. In zebrafish and Japanese eels, Amh is characterized as a “spermatogenesis-preventing substance” [94][98]. Studies of zebrafish have demonstrated that Amh counteracts gonadotropin-induced effects on Leydig cell steroidogenesis [94], inhibits Sertoli cell pro-differentiation Igf3, and stimulates inhibitory factors such as inhibin-α and prostaglandin E2 (Figure 6) [95]. These data clearly demonstrate that Amh inhibits spermatogonium differentiation through modulation of growth factor production and suppression of Leydig cell function [94][95], maintaining spermatogonia in an undifferentiated state (Figure 6).
The other well-known studied growth factor, Igf3, has been shown to be expressed exclusively in the gonad tissue in several teleost species [12]. In the testes, Igf3 has been detected in Sertoli cells [11][99], as well as in undifferentiated and differentiated spermatogonia, spermatocytes, and spermatids of tilapia [99]. There is evidence that FSH stimulates Igf3, which, in turn, promotes spermatogonial proliferation and differentiation in zebrafish testes (Figure 6) [11]. A recent study has shown that FSH-stimulated Igf3 release activates β-cateninc signaling in type A spermatogonia to stimulate their differentiation (Figure 6) [100].
Accumulated evidence in zebrafish demonstrates that FSH stimulates spermatogonial differentiation by down-regulating Amh and activating β-catenin signaling via Igf3. In addition, FSH promotes spermatogonial self-renewal through Wnt5a and the non-canonical Wnt pathways [12]. This balanced regulation could counteract depletion of Aund spermatogonia while promoting spermatogonial differentiation (Figure 6).
References
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411.
- Fawcett, D.W. Intercellular bridges. Exp. Cell Res. 1961, 8, 174–187.
- Grier, H.J. Comparative organization of Sertoli cells including the Sertoli cell barrier. The Sertoli Cell 1993, 703–739.
- Loir, M. Trout steroidogenic testicular cells in primary culture: II. Steroidogenic activity of interstitial cells, Sertoli cells, and spermatozoa. Gen. Comp. Endocrinol. 1990, 78, 388–398.
- Maekawa, M.; Kamimura, K.; Nagano, T. Peritubular Myoid Cells in the Testis: Their Structure and Function. Arch. Histol. Cytol. 1996, 59, 1–13.
- França, L.R.; Nóbrega, R.H.; Morais, R.D.V.S.; Assis, L.H.D.C.; Schulz, R.W. Sertoli cell structure and function in anamniote vertebrates. In Sertoli Cell Biology; Academic Press: Oxford, UK, 2015; pp. 385–407.
- Hess, R.A.; De Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. In Molecular Mechanisms in Spermatogenesis; Springer: New York, NY, USA, 2009; pp. 1–15.
- Figueiredo, A.F.A.; França, L.R.; Hess, R.A.; Costa, G.M.J. Sertoli cells are capable of proliferation into adulthood in the transition region between the seminiferous tubules and the rete testis in Wistar rats. Cell Cycle 2016, 15, 2486–2496.
- Callard, G.V. Endocrinology of Leydig cells in nonmammalian vertebrates. Leydig Cell 1996, 308–331.
- Wistuba, J.; Schlatt, S. Transgenic mouse models and germ cell transplantation: Two excellent tools for the analysis of genes regulating male fertility. Mol. Genet. Metab. 2002, 77, 61–67.
- Nóbrega, R.H.; De Souza Morais, R.D.V.; Crespo, D.; De Waal, P.P.; De França, L.R.; Schulz, R.W.; Bogerd, J. Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology 2015, 156, 3804–3817.
- Safian, D.; Bogerd, J.; Schulz, R.W. Regulation of spermatogonial development by Fsh: The complementary roles of locally produced Igf and Wnt signaling molecules in adult zebrafish testis. Gen. Comp. Endocrinol. 2019, 284, 1–11.
- De Rooij, D.G.; Russell, L.D. All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 2000, 21, 776–798.
- De Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reproduction-Cambridge- 2001, 121, 347–354.
- De Rooij, D.G. Rapid expansion of the spermatogonial stem cell tool box. Proc. Natl. Acad. Sci. USA 2006, 103, 7939–7940.
- De Rooij, D.G. Regulation of spermatogonial stem cell behavior in vivo and in vitro. Anim. Reprod. 2018, 3, 130–134.
- Ehmcke, J.; Wistuba, J.; Schlatt, S. Spermatogonial stem cells: Questions, models and perspectives. Hum. Reprod. Update 2006, 12, 275–282.
- Nóbrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de França, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e0012808.
- De Cuevas, M.; Matunis, E.L. The stem cell niche: Lessons from the Drosophila testis. Development 2011, 138, 2861–2869.
- Lacerda, S.M.S.N.; Aponte, P.M.; Costa, G.M.J.; Segatelli, T.M. An overview of spermatogonial stem cell physiology, niche and transplantation in fish. Anim. Reprod. 2012, 9, 798–808.
- De Siqueira-Silva, D.H.; da Silva Rodrigues, M.; Nóbrega, R.H. Testis structure, spermatogonial niche and Sertoli cell efficiency in Neotropical fish. Gen. Comp. Endocrinol. 2019, 273, 218–226.
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104.
- Fuchs, E.; Tumbar, T.; Guasch, G. Socializing with the neighbors: Stem cells and their niche. Cell 2004, 116, 769–778.
- Smith, A. A glossary for stem-cell biology. Nature 2006, 441, 1060.
- McLean, D.J. Spermatogonial stem cell transplantation and testicular function. Cell Tissue Res. 2005, 322, 21–31.
- Hess, R.A.; Cooke, P.S.; Hofmann, M.-C.; Murphy, K.M. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle 2006, 5, 1164–1170.
- Cooke, P.S.; Hess, R.A.; Simon, L.; Schlesser, H.N.; Carnes, K.; Tyagi, G.; Hofmann, M.C.; Murphy, K.M. The transcription factor Ets-related molecule (ERM) is essential for spermatogonial stem cell maintenance and self-renewal. Anim. Reprod. 2018, 3, 98–107.
- Oatley, J.M.; Oatley, M.J.; Avarbock, M.R.; Tobias, J.W.; Brinster, R.L. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 2009, 136, 1191–1199.
- Mäkelä, J.-A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 1, 169–187.
- Mayerhofer, A. Peritubular cells of the human testis: Prostaglandin E2 and more. Andrology 2019, 1–5.
- Chiarini-Garcia, H.; Hornick, J.R.; Griswold, M.D.; Russell, L.D. Distribution of type A spermatogonia in the mouse is not random. Biol. Reprod. 2001, 65, 1179–1185.
- Chiarini-Garcia, H.; Raymer, A.M.; Russell, L.D. Non-random distribution of spermatogonia in rats: Evidence of niches in the seminiferous tubules. Reproduction-Cambridge- 2003, 126, 669–680.
- Yoshida, S.; Sukeno, M.; Nabeshima, Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 2007, 317, 1722–1726.
- De Rooij, D.G.; Griswold, M.D. Questions about spermatogonia posed and answered since 2000. J. Androl. 2012, 33, 1085–1095.
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 2014, 14, 658–672.
- Kitadate, Y.; Jörg, D.J.; Tokue, M.; Maruyama, A.; Ichikawa, R.; Tsuchiya, S.; Segi-Nishida, E.; Nakagawa, T.; Uchida, A.; Kimura-Yoshida, C. Competition for mitogens regulates spermatogenic stem cell homeostasis in an open niche. Cell Stem Cell 2019, 24, 79–92.
- De Siqueira-Silva, D.H.; Saito, T.; dos Santos-Silva, A.P.; da Silva Costa, R.; Psenicka, M.; Yasui, G.S. Biotechnology applied to fish reproduction: Tools for conservation. Fish Physiol. Biochem. 2018, 44, 1469–1485.
- Schulze, C. Response of the human testis to long-term estrogen treatment: Morphology of Sertoli cells, Leydig cells and spermatogonial stem cells. Cell Tissue Res. 1988, 251, 31–43.
- Schulze, C. Morphological characteristics of the spermatogonial stem cells in man. Cell Tissue Res. 1979, 198, 191–199.
- Nakamura, S.; Kobayashi, K.; Nishimura, T.; Higashijima, S.I.; Tanaka, M. Identification of germline stem cells in the ovary of the teleost medaka. Science 2010, 328, 1561–1563.
- De Paiva Camargo, M.; Cassel, M.; Oliveira de Jesus, L.W.; Nóbrega, R.H.; Borella, M.I. Characterization of undifferentiated spermatogonia and the spermatogonial niche in the lambari fish Astyanax altiparanae. Theriogenology 2017, 96, 97–102.
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302.
- Majhi, S.K.; Hattori, R.S.; Yokota, M.; Watanabe, S.; Strüssmann, C.A. Germ cell transplantation using sexually competent fish: An approach for rapid propagation of endangered and valuable germlines. PLoS ONE 2009, 4, e0006132.
- Lacerda, S.M.S.N.; Batlouni, S.R.; Costa, G.M.J.; Segatelli, T.M.; Quirino, B.R.; Queiroz, B.M.; Kalapothakis, E.; França, L.R. A new and fast technique to generate offspring after germ cells transplantation in adult fish: The nile tilapia (Oreochromis niloticus) model. PLoS ONE 2010, 5, e0010740.
- Ye, H.; Li, C.-J.; Yue, H.-M.; Du, H.; Yang, X.-G.; Yoshino, T.; Hayashida, T.; Takeuchi, Y.; Wei, Q.-W. Establishment of intraperitoneal germ cell transplantation for critically endangered Chinese sturgeon Acipenser sinensis. Theriogenology 2017, 94, 37–47.
- Hattori, R.S.; Yoshinaga, T.T.; Katayama, N.; Hattori-Ihara, S.; Tsukamoto, R.Y.; Takahashi, N.S.; Tabata, Y.A. Surrogate production of Salmo salar oocytes and sperm in triploid Oncorhynchus mykiss by germ cell transplantation technology. Aquaculture 2019, 506, 238–245.
- Okutsu, T.; Shikina, S.; Kanno, M.; Takeuchi, Y.; Yoshizaki, G. Production of trout offspring from triploid salmon parents. Science 2007, 317, 1517.
- Lacerda, S.M.; Dos, S.N.; Costa, G.M.J.; de França, L.R. Biology and identity of fish spermatogonial stem cell. Gen. Comp. Endocrinol. 2014, 207, 56–65.
- Nagano, M.C.; Yeh, J.R. The identity and fate decision control of spermatogonial stem cells: Where is the point of no return. In Current Topics in Developmental Biology; Academic Press: Oxford, UK, 2013; Volume 102, pp. 61–95. ISBN 0070-2153.
- Yano, A.; Suzuki, K.; Yoshizaki, G. Flow-Cytometric Isolation of Testicular Germ Cells from Rainbow Trout (Oncorhynchus mykiss) Carrying the Green Fluorescent Protein Gene Driven by Trout vasa Regulatory Regions. Biol. Reprod. 2008, 78, 151–158.
- Okutsu, T.; Suzuki, K.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc. Natl. Acad. Sci. USA 2006, 103, 2725–2729.
- Buaas, F.W.; Kirsh, A.L.; Sharma, M.; McLean, D.J.; Morris, J.L.; Griswold, M.D.; de Rooij, D.G.; Braun, R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004, 36, 647–652.
- Panda, R.P.; Barman, H.K.; Mohapatra, C. Isolation of enriched carp spermatogonial stem cells from Labeo rohita testis for in vitro propagation. Theriogenology 2011, 76, 241–251.
- Ozaki, Y.; Saito, K.; Shinya, M.; Kawasaki, T.; Sakai, N. Evaluation of Sycp3, Plzf and Cyclin B3 expression and suitability as spermatogonia and spermatocyte markers in zebrafish. Gene Expr. Patterns 2011, 11, 309–315.
- Mohapatra, C.; Barman, H.K. Identification of promoter within the first intron of Plzf gene expressed in carp spermatogonial stem cells. Mol. Biol. Rep. 2014, 41, 6433–6440.
- Gautier, A.; Bosseboeuf, A.; Auvray, P.; Sourdaine, P. Maintenance of Potential Spermatogonial Stem Cells In Vitro by GDNF Treatment in a Chondrichthyan Model (Scyliorhinus canicula L.)1. Biol. Reprod. 2014, 91, 91–105.
- Bellaiche, J.; Lareyre, J.-J.; Cauty, C.; Yano, A.; Allemand, I.; Le Gac, F. Spermatogonial Stem Cell Quest: nanos2, Marker of a Subpopulation of Undifferentiated a Spermatogonia in Trout Testis1. Biol. Reprod. 2014, 90, 1–6.
- Nayak, S.; Ferosekhan, S.; Sahoo, S.K.; Sundaray, J.K.; Jayasankar, P.; Barman, H.K. Production of fertile sperm from in vitro propagating enriched spermatogonial stem cells of farmed catfish, Clarias batrachus. Zygote 2016, 24, 814–824.
- Shang, M.; Su, B.; Lipke, E.A.; Perera, D.A.; Li, C.; Qin, Z.; Li, Y.; Dunn, D.A.; Cek, S.; Peatman, E. Spermatogonial stem cells specific marker identification in channel catfish, Ictalurus punctatus and blue catfish, I. furcatus. Fish Physiol. Biochem. 2015, 41, 1545–1556.
- Lacerda, S.M.S.N.; Martinez, E.R.M.; Mura, I.L.D.D.; Doretto, L.B.; Costa, G.M.J.; Silva, M.A.; Digmayer, M.; Nóbrega, R.H.; França, L.R. Duration of spermatogenesis and identification of spermatogonial stem cell markers in a Neotropical catfish, Jundiá (Rhamdia quelen). Gen. Comp. Endocrinol. 2019, 273, 249–259.
- Meng, X.; Lindahl, M.; Hyvönen, M.E.; Parvinen, M.; de Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493.
- Nakagawa, T.; Sharma, M.; Nabeshima, Y.; Braun, R.E.; Yoshida, S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010, 328, 62–67.
- Costa, G.M.J.; Avelar, G.F.; Guimarães, D.A.; França, L.R. Postnatal testis development in the collared peccary (Tayassu tajacu), with emphasis on spermatogonial stem cells markers and niche. Gen. Comp. Endocrinol. 2019, 273, 98–107.
- Savvulidi, F.; Ptacek, M.; Savvulidi Vargova, K.; Stadnik, L. Manipulation of spermatogonial stem cells in livestock species. J. Anim. Sci. Biotechnol. 2019, 10, 46–63.
- Santos Nassif Lacerda, S.M.; Costa, G.M.J.; da Silva, M.D.A.; Almeida Campos-Junior, P.H.; Segatelli, T.M.; Peixoto, M.T.D.; Resende, R.R.; de França, L.R. Phenotypic characterization and in vitro propagation and transplantation of the Nile tilapia (Oreochromis niloticus) spermatogonial stem cells. Gen. Comp. Endocrinol. 2013, 192, 95–106.
- Nakajima, S.; Hayashi, M.; Kouguchi, T.; Yamaguchi, K.; Miwa, M.; Yoshizaki, G. Expression patterns of gdnf and gfrα1 in rainbow trout testis. Gene Expr. Patterns 2014, 14, 111–120.
- Froschauer, A.; Khatun, M.M.; Sprott, D.; Franz, A.; Rieger, C.; Pfennig, F.; Gutzeit, H.O. oct4-EGFP reporter gene expression marks the stem cells in embryonic development and in adult gonads of transgenic medaka. Mol. Reprod. Dev. 2013, 80, 48–58.
- Sánchez-Sánchez, A.V.; Camp, E.; Mullor, J.L. Fishing pluripotency mechanisms in vivo. Int. J. Biol. Sci. 2011, 7, 410–417.
- Wang, D.; Manali, D.; Wang, T.; Bhat, N.; Hong, N.; Li, Z.; Wang, L.; Yan, Y.; Liu, R.; Hong, Y. Identification of pluripotency genes in the fish medaka. Int. J. Biol. Sci. 2011, 7, 440–451.
- Baumann, K. A gated exit from pluripotency. Nat. Rev. Mol. Cell Biol. 2013, 14, 324.
- Lengner, C.J.; Camargo, F.D.; Hochedlinger, K.; Welstead, G.G.; Zaidi, S.; Gokhale, S.; Scholer, H.R.; Tomilin, A.; Jaenisch, R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell 2007, 1, 403–415.
- Hayashi, M.; Ichida, K.; Sadaie, S.; Miwa, M.; Fujihara, R.; Nagasaka, Y.; Yoshizaki, G. Establishment of novel monoclonal antibodies for identification of type A spermatogonia in teleosts†. Biol. Reprod. 2019, 101, 478–491.
- Ichida, K.; Hayashi, M.; Miwa, M.; Kitada, R.; Takahashi, M.; Fujihara, R.; Boonanuntanasarn, S.; Yoshizaki, G. Enrichment of transplantable germ cells in salmonids using a novel monoclonal antibody by magnetic-activated cell sorting. Mol. Reprod. Dev. 2019, 86, 1810–1821.
- Ichida, K.; Kawamura, W.; Miwa, M.; Iwasaki, Y.; Kubokawa, T.; Hayashi, M.; Yazawa, R.; Yoshizaki, G. Specific visualization of live type A spermatogonia of Pacific bluefin tuna using fluorescent dye-conjugated antibodies†. Biol. Reprod. 2019, 100, 1637–1647.
- Kobayashi, T.; Kajiura-Kobayashi, H.; Nagahama, Y. A novel stage-specific antigen is expressed only in early stages of spermatogonia in Japanese eel, Anguilla japonica testis. Mol. Reprod. Dev. 1998, 51, 355–361.
- Yano, A.; Von Schalburg, K.; Cooper, G.; Koop, B.F.; Yoshizaki, G. Identification of a molecular marker for type A spermatogonia by microarray analysis using gonadal cells from pvasa-GFP transgenic rainbow trout (Oncorhynchus mykiss). Mol. Reprod. Dev. Inc. Gamete Res. 2009, 76, 246–254.
- Nagasawa, K.; Shikina, S.; Takeuchi, Y.; Yoshizaki, G. Lymphocyte antigen 75 (Ly75/CD205) is a surface marker on mitotic germ cells in rainbow trout. Biol. Reprod. 2010, 83, 597–606.
- Nagasawa, K.; Miwa, M.; Yazawa, R.; Morita, T.; Takeuchi, Y.; Yoshizaki, G. Characterization of lymphocyte antigen 75 (Ly75/CD205) as a potential cell-surface marker on spermatogonia in Pacific bluefin tuna Thunnus orientalis. Fish. Sci. 2012, 78, 791–800.
- Aoki, Y.; Nakamura, S.; Ishikawa, Y.; Tanaka, M. Expression and syntenic analyses of four nanos genes in medaka. Zoolog. Sci. 2009, 26, 112–118.
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 1981, 50, 465–495.
- McLachlan, R.I.; Wreford, N.G.; O’donnell, L.; De Kretser, D.M.; Robertson, D.M. The endocrine regulation of spermatogenesis: Independent roles for testosterone and FSH. J. Endocrinol. 1996, 148, 1–9.
- Panneerdoss, S.; Chang, Y.-F.; Buddavarapu, K.C.; Chen, H.-I.H.; Shetty, G.; Wang, H.; Chen, Y.; Kumar, T.R.; Rao, M.K. Androgen-responsive microRNAs in mouse Sertoli cells. PLoS ONE 2012, 7, e0041146.
- Shirakawa, T.; Yaman-Deveci, R.; Tomizawa, S.; Kamizato, Y.; Nakajima, K.; Sone, H.; Sato, Y.; Sharif, J.; Yamashita, A.; Takada-Horisawa, Y. An epigenetic switch is crucial for spermatogonia to exit the undifferentiated state toward a Kit-positive identity. Development 2013, 140, 3565–3576.
- Nagano, M.; Ryu, B.-Y.; Brinster, C.J.; Avarbock, M.R.; Brinster, R.L. Maintenance of mouse male germ line stem cells in vitro. Biol. Reprod. 2003, 68, 2207–2214.
- Ohta, T.; Miyake, H.; Miura, C.; Kamei, H.; Aida, K.; Miura, T. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol. Reprod. 2007, 77, 970–977.
- García-López, Á.; De Jonge, H.; Nóbrega, R.H.; De Waal, P.P.; Van Dijk, W.; Hemrika, W.; Taranger, G.L.; Bogerd, J.; Schulz, R.W. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 2010, 151, 2349–2360.
- García-Lopez, A.; Bogerd, J.; Granneman, J.C.M.; van Dijk, W.; Trant, J.M.; Taranger, G.L.; Schulz, R.W. Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology 2009, 150, 357–365.
- Chauvigné, F.; Zapater, C.; Gasol, J.M.; Cerdà, J. Germ-line activation of the luteinizing hormone receptor directly drives spermiogenesis in a nonmammalian vertebrate. Proc. Natl. Acad. Sci. USA 2014, 111, 1427–1432.
- Safian, D.; Ryane, N.; Bogerd, J.; Schulz, R.W. Fsh stimulates Leydig cell Wnt5a production, enriching zebrafish type A spermatogonia. J. Endocrinol. 2018, 239, 351–363.
- Assis, L.H.C.; Crespo, D.; Morais, R.D.V.S.; França, L.R.; Bogerd, J.; Schulz, R.W. INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio). Cell Tissue Res. 2016, 363, 579–588.
- Planas, J.V.; Swanson, P.; Dickhoff, W.W. Regulation of testicular steroid production in vitro by gonadotropins (GTH I and GTH II) and cyclic AMP in coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 1993, 91, 8–24.
- Crespo, D.; Assis, L.H.C.; Furmanek, T.; Bogerd, J.; Schulz, R.W. Expression profiling identifies Sertoli and Leydig cell genes as Fsh targets in adult zebrafish testis. Mol. Cell. Endocrinol. 2016, 437, 237–251.
- Sambroni, E.; Rolland, A.D.; Lareyre, J.J.; Le Gac, F. Fsh and Lh have common and distinct effects on gene expression in rainbow trout testis. J. Mol. Endocrinol. 2013, 50, 1–18.
- Skaar, K.S.; Nobrega, R.H.; Magaraki, A.; Olsen, L.C.; Schulz, R.W.; Male, R. Proteolytically activated, recombinant Anti-Müllerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology 2011, 152, 3527–3540.
- Morais, R.D.V.S.; Crespo, D.; Nóbrega, R.H.; Lemos, M.S.; van de Kant, H.J.G.; de França, L.R.; Male, R.; Bogerd, J.; Schulz, R.W. Antagonistic regulation of spermatogonial differentiation in zebrafish (Danio rerio) by Igf3 and Amh. Mol. Cell. Endocrinol. 2017, 454, 112–124.
- Adolfi, M.C.; Nakajima, R.T.; Nóbrega, R.H.; Schartl, M. Intersex, hermaphroditism, and gonadal plasticity in vertebrates: Evolution of the Müllerian duct and Amh/Amhr2 signaling. Annu. Rev. Anim. Biosci. 2019, 7, 149–172.
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish–multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107.
- Miura, T.; Miura, C.; Konda, Y.; Yamauchi, K. Spermatogenesis-preventing substance in Japanese eel. Development 2002, 129, 2689–2697.
- Li, M.; Liu, X.; Dai, S.; Xiao, H.; Qi, S.; Li, Y.; Zheng, Q.; Jie, M.; Cheng, C.H.K.; Wang, D. Regulation of spermatogenesis and reproductive capacity by Igf3 in tilapia. Cell. Mol. Life Sci. 2020, 1–18.
- Safian, D.; Bogerd, J.; Schulz, R.W. Igf3 activates β-catenin signaling to stimulate spermatogonial differentiation in zebrafish. J. Endocrinol. 2018, 238, 245–257.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




