
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Suresh Mickymaray | + 10689 word(s) | 10689 | 2021-06-02 10:26:55 | | | |
| 2 | Vivi Li | -6046 word(s) | 4643 | 2021-06-10 04:54:55 | | |
Video Upload Options
The prevalence of fungal infections is growing at an alarming pace and the pathogenesis is still not clearly understood. Recurrence of these fungal diseases is often due to their evolutionary avoidance of antifungal resistance. The development of suitable novel antimicrobial agents for fungal diseases continues to be a major problem in the current clinical field. Hence, it is urgently necessary to develop surrogate agents that are more effective than conventional available drugs. Among the remarkable innovations from earlier investigations on natural-drugs, flavonoids are a group of plant-derived substances capable of promoting many valuable effects on humans. The identification of flavonoids with possible antifungal effects at small concentrations or in synergistic combinations could help to overcome this problem. A combination of flavonoids with available drugs is an excellent approach to reduce the side effects and toxicity.
1. Introduction
Fungal illness often can be fatal, killing more than 1.5 million a year, and such illnesses have an effect on over a billion peoples in a year. Nevertheless, public health authorities have continued to neglect the issue, although the majority of deaths are from fungal infectious diseases. The severe fungal infections often arise because of other health issues, including acquired immunodeficiency syndrome (AIDS), cancer, asthma, diabetes, organ transplantation, and treatment with corticosteroids [1][2]. Fungal infections have augmented constantly in the current decennium, mainly in immunocompromised hosts or hospitalized individuals with severe underlying infections [3]. Yeasts are large, widespread opportunistic agents in fungal infectious diseases, and various fungal pathogens have been developed in the past decennium [4]. Among the fungal infections, Candida, Aspergillus, Pneumocystis, and Cryptococcus are the main threatening agents globally due to the severity and higher incidence of the diseases [5][6]. It is projected worldwide that these fungal species produce, annually, at least 1.4 million fatalities [7][8]. Candida spp. is the most isolated yeast among systemic fungal infections [9][10]. Candida is a genus of eukaryotic fungus comprised of 17 species out of 150, which are well-known causative agents of candidiasis in humans [11]. According to the National Network of Health Security, Candida spp. are the third most widespread causative agent of blood culture infections (15%) connected to intensive care units, after other common bacterial pathogens [12][13]. Candida albicans is the most ubiquitous species globally (50–70%), which produces more infectious diseases than the total occurrence of infections produced by C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei [9][14]. These yeasts primarily cause superficial and systemic fungal infections that include biofilm-associated infections candidaemia, and fungemia in patients with malignancies [9][15][16]. Aspergillus infections are another foremost infection occurring in recipients of hematopoietic stem cell transplants. About 30% of individuals may die from invasive aspergillosis, and the remaining 50% of deaths may occur by candidemia [7][17]. Cryptococcus spp. is another medically noteworthy yeast species, consisting of 40 species; among them, C. gattii, and C. neoformans are the most clinically applicable [18]. In addition, C. albidus and C. laurentii are developing pathogens that are participated in various kinds of infectious diseases [19][20][21]. Cryptococcosis is greatly connected with AIDS and meningitis [22]. This infection normally takes place exogenously through breathing or by direct inoculation into the host tissue [4].
The growing resistance of microbes against exiting antifungal drugs is one of the main issues among researchers and clinicians. Pathogenic fungi, viruses, bacteria, and protozoa are more challenging to treat with the existing drugs due to the development of resistance [23][24]. Numerous investigations related to antimicrobial resistance estimated that the mortality rate may go above 10 million by 2050, possibly leading to higher mortality when compared to malignancies and metabolic diseases [25][26][27][28][29]. The resistance of pathogenic fungi to available antibiotics has developed into a global epidemic. Therapeutic agents for fungal infections are negligible when related to therapeutic agents for bacterial infections [30][31]. In order to heal fungal infections, four categories of antifungal drugs are often offered; viz., polyenes (amphotericin B, nystatin, candicidin, pimaricin, methyl partricin, trichomycin), azoles (fluconazole, itraconazole, ketoconazole, miconazole, clotrimazole, voriconazole, posaconazole, ravuconazole), echinocandins (caspofungins, micafungin, and anidulafungin), and flucytosine (5-fluorocytosine). However, those antifungal agents are only partially effective, and many of them produce several complications to host tissues. Based on a recent therapeutic search, limited antifungal agents have only been structurally and systematically elucidated in the past 30 years [28][32].
The development of resistance is habitually occurring by antifungal agents that usually bind with cell walls or biosynthetic pathways. For instance, there has been elevated use of fluconazole and amphotericin B, owing to their effectiveness and low toxicity and binding potential toward the membranes of fungal pathogens, consequently stimulating drug resistance [3][33]. A. fumigatus and C. krusei are fundamentally resistant to most azole class drugs; viz., fluconazole, itraconazole, voriconazole, and posaconazole. Similarly, Cryptococcus neoformans are resistant to fluconazole and echinocandins [7][34][35]. Hence, it is an urgent need to investigate novel drugs that have greater anti-fungal activity. The approaches of traditional plant-based medicine or bioactive natural products are great, as such therapeutic medicine can better the prevailing fungal treatments with lesser side effects [28][36][37][38].
2. Antifungal Activities of Flavonoids
The screening of antifungal flavonoids from plants has been assayed by using broth dilution, spore germination, and agar well or the disk diffusion. Derrone and licoflavone C extracted from Retama raetam, which has potent antifungal effects against Candida spp. with minimum inhibitory concentrations (MIC) of 7.81 and 15.62 μg/mL, correspondingly [39]. Papyriflavonol A acquired from Broussonetia papyrifera that verified as antifungal agents against C. albicans with a MIC of 25 μg/mL [40]. A plant-derived flavonoid, Quercetin-3-O-rutinosides had beneficial effects on C. albicans and C. krusei that exhibited with MICs of 16 and 32 μg/mL respectively [41]. Two distinguished flavonoids, 5,7,4′-trihydroxy-8-methyl-6-(3-methyl-[2-butenyl])-2S- flavanone and 7-hydroxy-3′,4′-methylene dioxy flavan obtained from Eysenhardtia texana and Termanalia bellerica, which possess potential antifungal properties against A. flavus with MICs of 256 and 64 μg/mL respectively [42]. Renowned flavonoids such as quercetin, myricetin, baicalein (from Scutellaria baicalensis), gallotannin (from Syzygium cordatum), apigenin and kaempferol (from propolis) isolated and reported as potential anti-candidal properties [43][44][45]. In addition, coumarins and lignans have also presented antifungal effects against numerous dermatophyte species [46][47][48][49][50][51][52]. Flavonoids and catechins acquired from Brazilian traditional medicinal plants, Eugenia dysenterica, and Pouteria ramiflora that have shown potential antifungal activities against C. tropicalis, C. famata, C. krusei, C. guilliermondii, and C. parapsilosis [53].
Various folkloric medicinal plants contain various fractions of flavonoids that show antifungal properties. Ocotea odorifera contain ellagitannins, has reported as a fungistatic potential against C. parapsilosis [54]. Sanguiin H-6 and lambertianin C and isolated from raspberry (Rubus idaeus L.) fruit reported as antifungal effects against Geotrichum candidum [55]. Acacia mearnsii contains encapsulated tannins that inhibit the effects against A. niger and C. albicans [56]. Propolis and its high flavonoid content have antifungal activity against dermatophytes and Candida spp. Exclusively, propolis contains a flavonol, galangin, which has been demonstrated to have antifungal activities against Cladosporium sphaerospermum, Penicillium digitatum, A. tamarii, A. flavus, and P. italicum [57].
Nobiletin, langeritin and hesperidin have extracted from the peels of tangerine oranges and assayed for the activity towards Deuterophoma tracheiphila that exhibits promising antifungal activities [58]. The antifungal effects have also been reported in flavonoids extracted from citrus fruits after processing in industries and bergamot peel that averts the growth of S. cerevisiae [59]. Quercetin, naringenin is recognized to be potent inhibitors of C. albicans, and S. cerevisiae [60]. Chlorflavonin is the first chlorine-containing flavonoid type antifungal agent, produced by strains of A. candidus [61]. A recognized flavone, baicalein; and flavonol, myricetin have greater inhibitory effects on Candida sp., with MICs of 1.9–21 and 3.9–64 μg/mL, correspondingly [62].
The antifungal activity of 40 coumarins have studied against C. albicans, A. fumigatus, and F. solani, among them, osthenol and 4-acetetatecoumarin have demonstrated higher antifungal effects [63]. Petroleum ether extracts of Baccharis darwinii and Ferula foetida contain well-known coumarin, diversinin and 5, 8-dihydroxyumbelliprenin, which have confirmed antifungal activity against T. rubrum, T. interdigitale, T. mentagrophytes, and M. gypseum [46]. Phenylpropanoids are natural compounds that classified as coumarins, lignans and phenylpropanoic acid, often investigated due to their anti-candidal nature [3]. Scopoletin (coumarin), salicylaldehyde and anisyl alcohol (phenylpropanoic acids) have potential antifungal effects against C. albicans, with MICs of 25, 31, and 31 μg/mL correspondingly [64][65]. Similarly, antifungal activities have been described in hesperidin, neohesperidin, naringin which are normally isolated from the citrus fruits. These compounds have strong fungal inhibitory activity against P. expansum, F. semitectum, A. parasiticus, A. flavus [66].
Grapes are a rich source of flavonoids, and their pomaces largely help to avert the growth of Zygosaccharomyces bailii and Zygosaccharomyces rouxii [67]. Chilean grape pomace extract is recognized to have antifungal activity against Botrytus cinerea [68,69]. The growth of C. albicans could be averted by flavonoid extracts from Brazilian grapes [70]. Similarly, Eysenhardita texana has prenylated flavanones that have potential antifungal activity against C. albicans [71]. Flavanol is generally found in propolis that is also suggested to be used as antifungal agents [72]. Flavonoid extracts of Sida acuta Burm f. have shown a varying range of antifungal activity against C. albicans. The degree of MIC and Minimum fungicidal concentration of extracts have accounted for 0.078–0.625 mg/mL and 0.078–1.25 mg/mL, correspondingly [73]. Bitencourt et al. [74] demonstrated that the four flavonoids such as quercetin, ellagic acid, galangin, and genistein have shown the most potential antifungal property with MIC of 125, 250, 1000, 1000 µg/mL against Trichophyton rubrum, which is common species among the fungal associated dermatophytosis. This team has further reported the antifungal potential of flavonoids that have been recognized as FAS inhibitors which modulate the fatty acid synthesis gene expressions in T. rubrum. The crude and butanolic leaf extract of Terminalia catappa contain the active components of hydrolyzable tannins (punicalin, punicalagin), gallic acid, and flavonoid C-glycosides that exhibits antifungal activity against Candida sp. [75]. Similarly, crude and ethanol leaf extracts of Carya illinoensis contain gallic acid, ellagic acid, rutin, catechins and epicatechins that exhibits antifungal activity against different Candida strains with MIC range of 6.25–25 mg/mL [76].
Gallic acid is established to have potent antifungal property against Candida spp., and T. rubrum. Gallic acid is isolated from acetone fraction of Buchenavia tomentosa that inhibits the proliferation rate and disrupts 48 h-biofilm abruptly in C. albicans [77]. Ethyl acetate and butanolic extracts of Punica granatum contain ellagic acid, gallagic acid, punicalins, and punicalagins which show antifungal activities against C. albicans, C. neoformans, and A. fumigatus [78]. Curcumin is a renowned flavonoid present in turmeric, which has potential anti-candidal activity against various clinical isolates of C. albicans [79] and C. gattii [80]. Curcumin can decrease the colony width, sprouting, and sporulation of A. flavus and C. albicans [81]. Similarly, Curcumin-silver nanoparticles have also exhibited potential anti-candidal activity against Candida species acquired from clinical samples of infected HIV individuals with MIC range of 31.2–250 μg/mL [82]. All these findings strongly recommend that flavonoids exhibit a broad spectrum of antifungal activity against Candida spp., Aspergillus spp., Geotrichum spp., Cladosporium spp., Penicillium spp., Deuterophoma spp., Trichophyton spp., Trichophyton spp., Dermatophyte spp., and Fusarium spp.
3. Actions of Antifungal Flavonoids
Flavonoids have been extensively used for many centuries in the treatment of the range of human diseases. Flavonoids often inhibit fungal growth with various underlying mechanisms, including plasma membrane disruption, the induction of mitochondrial dysfunction, and inhibiting the following: cell wall formation, cell division, RNA and protein synthesis, and the efflux mediated pumping system (Figure 1).
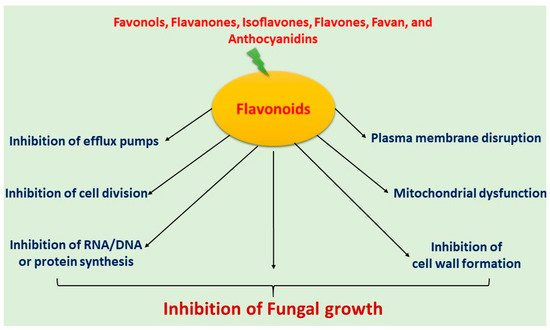
Figure 1. Mechanism of antifungal activity of flavonoids.
3.1. Induced Plasma Membrane Disruption
The ergosterols are a vital component for the manufacturing of cell membranes. Antifungal drugs normally inhibit the ergosterol biosynthesis, and the cell membrane’s integrity is perhaps disrupted, leading to leakage of intracellular components [83][84]. This inadequate formation or disruption of the plasma membrane leads to a lesion or membrane permeability changes [85]. Furthermore, excess production of reactive oxygen species (ROS) also causes severe oxidative stress to the cell, which results in the progressive membrane permeabilization, or injury to nucleic acids and oxidation of fatty acids and amino acids [86][87][88]. ROS often encounter the membrane lipids in C. albicans and generate lipid hydroperoxides; this is known as lipid peroxidation [89].
Lipid peroxidation has been demonstrated to disturb the lipid bilayer and alter membrane potentials, resulting in reduced fluidity, increased permeability, and disruption of phospholipids [90]. The relationship between ROS generation and the lipid bilayer leads to the synthesis of malondialdehyde, which is a chief marker of lipid peroxidation [24][91][92][93][94][95][96]. Apigenin has exerted antioxidant and antifungal activity against C. albicans, C. parapsilosis, Malassezia furfur, T. rubrum, and T. beigelii all with the MIC of 5 µg/mL. Antioxidant potential of the flavonoid inhibits biofilm formation and stimulates membrane disturbances, resulting in the reduction of cell size and leakage of intracellular components [97]. In the previous study, LicoA demonstrated antifungal activities against T. rubrum with MIC of 11.52 μM, and the orientation of genes connected to the pathway of ergosterol biosynthesis [98]. In an earlier study, prenylflavanone 8PP obtained from Dalea elegans, had potential antifungal activity against C. albicans, C. glabrata, C. krusei, C. neoformans, and T. mentagrophytes [99]. In this study, prenylflavanone 8PP potentially inhibited the biofilms of sensitive and azole-resistant C. albicans at 100 μM through the gathering and elevation of endogenous ROS and reactive nitrogen intermediates [100][101][102][103][104][105][106][107].
Similarly, Baicalein has been isolated from Scutellaria baicalensis, which shows inhibitory effects towards Candida spp. when used in synergetic mixture with flucanazole at MIC of 64 μg/mL [45][108][109]. Baicalein has induced the apoptosis through alteration in the membrane potentials of mitochondria and elevates intracellular ROS and upstream regulation of redox-related genes [110]. In another study, baicalein presented antifungal activities toward T. rubrum, C. albicans, T. mentagrophytes, and A. fumigatus with MICs of 120, 30, 60, and 230 μM respectively [111]. Baicalein has induced concentration-dependent ROS generation, deformation of membrane structure, and efflux of a cotton-like constituents that are alleged to degenerate cytosol in fungal bodies of T. rubrum, T. mentagrophytes, A. fumigatus, and C. albicans [111]. However, Kang et al. [109] reported controversial outcomes, including that antifungal screening of baicalein in C. krusei isolates showed higher alteration in the mitochondrial homeostasis without elevating the intracellular ROS, thereby causing apoptosis [109]. Antifungal activities of fisetin inhibit the growth of C. neoformans, C. gattii, M. gypseum, T. mentagrophytes, T. rubrum, and T. tonsurans with MIC range of 4–128 µg/mL. In this study, reductions of ergosterol levels and structural alterations were detected in C. gattii [112][113].
Fatty acid synthase is a significant enzyme essential for endogenous fatty acid synthesis in the membrane of fungi, indicating it as a potential target for novel antifungal drugs [114]. Quercetin has been reported to have individual or synergic antifungal properties with flucanazole, which is recognized as an inhibitor of fatty acid synthase. The inhibitory effects of quercetin and fluconazole were reported as MICs of 125 and 63 μg/mL against T. rubrum [115]. Likewise, catechin or epigallocatechin gallate have also shown synergic antifungal effects with the MIC values of 16 and 1 µg/mL respectively. These active flavonoids induce the activation of phosphatidylserine, which inhibits fatty acid synthase. In addition, they stimulate the intracellular accumulation of ROS, structural modifications, apoptosis, mitochondrial depolarization, and fragmentation of DNA in C. tropicalis [70]. Isoquercitrin has also shown antifungal activities against C. albicans, M. furfur, C. parapsilosis, T. rubrum, and T. beigelii with MIC values of 2.5–5.0 μg/mL through inhibition of fatty acid synthase and plasma membrane disruption [116].
3.2. Inhibition of Cell Wall Formation
The cell walls of fungi are primarily composed of β-glucans and chitin. The antifungal mechanism has been based on cell wall deformation which is caused by the inhibition of the synthesis of those compounds [84][117]. Glabridin is a chief active isoflavane isolated from Glycyrrhiza glabra, and has significant antifungal activities against C. albicans, C. tropicalis C. neoformans, and C. glabratas with MIC values ranging from 16 to 64 µg/mL. The antifungal process is achieved based on the cell wall deformation which includes the remarkable decreasing of cell size and increasing membrane permeability [118]. Similarly, glabridin treatment enhances the expression of various genes in C. glabrata which participate in the fragmentation of DNA (chromatin condensation) resulting in apoptosis [119]. These deformations of the cell wall normally occur due to the presence of the prenylation of glabridin [119]. Antifungal effects of pedalitin (5, 6, 3’,4’ tetrahydroxy-7-methoxyflavone) have been reported against several strains of C. albicans and Cryptococcus spp. [120]. An animal model study against disseminated Candidiasis showed epigallocatechin-o-gallate’s synergistic interaction with amphotericin B against C. albicans [177]. Infected animals administered with mixed doses of epigallocatechin-o-gallate and amphotericin B exhibited an augmented survival rate compared to animals administered with amphotericin B. The results show that epigallocatechin-o-gallate exclusively inhibits the hyphal formation and ergosterol synthesis in C. albicans [68]. The investigations of propidium iodide assay and artificial membrane permeability study specified that pedalitin stimulates the elevation of permeability and physical alarm of the plasma membrane, permitting the diffusion of molecules smaller than about 3.3 nm. These cell wall deformations and the membrane damage are generally promoted by pedalitin, which contributes to malfunctions of the membrane that causes depolarization, K+ leakage, and reduction in membrane fluidity, eventually leading to cell death [57][121].
3.3. Induced Mitochondrial Dysfunction
Inhibition of the mitochondrial electron transport chain (ETC) leads to diminishing membrane potential. This inhibition generally takes place in the ETC by inhibition of proton pumps, which reduces ATP synthesis, and thus, cell death [117]. Wogonin (5,7-dihydroxy-8-methoxy flavone) showed antifungal activity against A. fumigates, T. rubrum, and T. mentagrophytes with MICs of 230, 60, and 60 µM respectively. The treatment with wogonin induces accumulation of ROS in mitochondria and causes a decreased membrane potential and reducing ATP synthesis and eventually contraction or cracking of fungal filaments [111]. Baicalein inhibits biofilm formation in a dose-dependent manner from 4 to 32 µg/mL. The results of confocal scanning laser microscopy, flow cytometry, and transmission-electron-microscopy analysis have shown baicalein treatment reduces cell surface hydrophobicity and mRNA expression, and elevates apoptosis that is connected to the failure of mitochondrial membrane potential [122]. Similarly, quercetin, resveratrol, and curcumin modulate mitochondrial functions by inhibiting oxidative phosphorylation through various mitochondrial enzymes, or by changing the generation of ROS in mitochondria and by modulating the activity of transcription factors which control mitochondrial proteins’ expression [123][124]. All these compounds exhibit pro-apoptotic functions, mediated by the ability to discharge of cytochrome c from mitochondria, or indirectly by upregulating pro-apoptotic proteins of Bcl-2 expressions and downregulating anti-apoptotic proteins [125][126]. Honey extract also contains a flavonoid that improves mitochondrial functions and decreases the vacuolization, adjusting the branching process connected with virulence. Honey extract induces alterations in the cell cycle, membrane integrity, functions of mitochondria, and biogenesis [127]. A synergistic study has also investigated the synergy between epigallocatechin gallate and conventional antimycotics agents, such as miconazole, fluconazole, and amphotericin B, against biofilms of C. albicans, C. glabrata, C. parapsilosis, C. kefyr, C. tropicalis, and C. krusei. Similarly, epigallocatechin gallate has described as an anti-candidal agent, which has been demonstrated through the mechanism of mitochondrial membrane dysfunction [128]. Likewise, Spondias tuberosa rich flavonoids elevate the levels of the superoxide anion via the lysosome, causing hyperpolarization in the mitochondrial membrane, so granting anti-Candida activity [129].
3.4. Inhibition of Cell Division
The inhibition of cell division generally causes inhibition of microtubule polymerization, which inhibits the mitotic spindle formation ([117]. Honey flavonoid extract inhibits the proliferation of C. albicans phenotypes, diminishes the infection, and reduce the distressing membrane integrity. This inhibition is measured by using flow cytometry and scanning electron microscopy analyses. Honey flavonoid extract affects the hyphal transition by decreasing the G0/G1 phase and increasing the G2/M phase [127]. Some flavonoids, such as apigenin, α-naphthoflavone, 3′-methoxy-4’-nitroflavone, and 2′-amino-3′-methoxy flavone, have various ligands of the aryl hydrocarbon receptor that inhibit the cell cycle [130]. Studies show that alizarin and chrysazin suppress biofilm formation in C. albicans, and effectively inhibit hyphal formation and inhibit the cell cycle [131]. Another study shows that magnolol and honokiol inhibit the growth of C. albicans through the Ras1-cAMP-Efg1 pathway. These compounds have potential inhibitory effects on the cell cycle and biofilm-formation-ability of C. albicans [132]. Rubus chingii is a well-known traditional Chinese medicinal plant that possesses flavonoid-rich compounds, known to have significant antimicrobial and antifungal activities. The crude extract of this plant synergistically interacts with fluconazole to inhibit C. albicans. The probable mechanism behind this synergistic interaction could be the cell cycle arrest at S phase in C. albicans. The crude extract containing flavonoids reduce the efflux of Cdr1 ABC transporter, which may be the reason for fluconazole resistance [133]. Similarly, daphnegiravone D, a prenylated flavonoid, has cytotoxic effects and significantly inhibits cell division. Systematically, daphnegiravone D arrests the G0/G1 phase and stimulates apoptosis, by reducing the expression of cyclin E1, CDK2, and CDK4, and promote the cleavage of caspase 3 and PARP [134].
3.5. Inhibition of Efflux Pumps
Efflux pumps are transporters present in most living cells, including fungi; they have the noteworthy function of removing toxic substances from the fungal body [135][136][137]. This transporter can detoxify a fungal cell through the removal of a drug being accumulated. The high efflux pump’s expression can lead to drug-resistance. Hence, inhibiting the efflux pumps is a crucial aim for reducing drug resistance [138][139]. A flavone, 7,4′-dimethoxy apigenin, inhibits the growth of C. albicans when synergistically combined with miconazole. This combination reduces ergosterol biosynthesis and inhibits drug efflux pumps with IC50 of 51.64 µg/mL [140]. Baicalein (5,6,7-trihydroxy flavone) is a flavone, isolated from Scutellaria baicalens, that has significant anticandidal activity with a MIC value of 26 mg/mL. This compound is well recognized as a lipooxygenase inhibitor or efflux pump inhibitor when in combination with fluconazole; it decreases the capacity of the cells to efflux out drugs [141][142]. Similarly, diorcinol D is another natural compound obtained from a lichen endophytic fungus, Aspergillus versicolor, that inhibits the efflux pump activity by decreasing the Cdr1 expression in C. albicans [143]. Curcumin from rhizomes of Curcuma longa is also another natural polyphenolic compound that modulates the efflux pump activity in Saccharomyces cerevisiae, and overexpresses the C. albicans ATP binding cassette (ABC) multidrug transporters, Candida drug resistance protein 1, and Candida drug resistance protein 2 [144][145]. Similarly, the quorum-sensing molecule farnesol is drug efflux a modulator that mediates through ABC multidrug transporters and synergizes with fluconazole, ketoconazole, miconazole, and amphotericin B in C. albicans. This synergistic interaction of quorum-sensing molecule farnesol with those antifungal drugs leads to ROS generation, which causes early apoptosis [146][147]. Naturally occurring flavones, such as apigenin, chrysin, baicalein, luteolin, tangeritin, scutellarein, 6-hydroxyflavone, and wogonin inhibit efflux mediated pumps that induce cell death in the fungi [45][148][149]. An isoflavone, sedonan A extracted from Dalea formosa, also inhibits efflux pumps in C. albicans and C. glabrata, and disturbs various intracellular transcription genes with MIC of 15 and 7.6 mg/mL, respectively [150]. Another isoflavone has been identified as dorsmanin isolated from dorstenia mannii that inhibits efflux pumps in C. albicans with a MIC of 64 mg/ml [151][152].
3.6. Inhibition of RNA/DNA and Protein Synthesis
The antifungal agent generally enters into the cell through active transport that reaches into the nucleus, and thus inhibits DNA, RNA, and protein synthesis. The inhibition of protein synthesis is well-recognized as an antifungal target [117][153]. For instance, 5-flurocytosine inhibits nucleic acid synthesis by the formation of fluorinated pyrimidine metabolites, which can cause a deficit of cytosine deaminase, resulting in the deregulation of the pyrimidine biosynthesis [154][155]. Similarly, Catechin inhibits C. albicans nucleic acid synthesis; analysis by flow cytometry shows that it exhibits the inhibition of FCS-induced hyphal formation; western blotting results also reveal that the treatment with catechin in the C. albicans reduces the hypha-specific gene expression in mitogen-activated protein kinase cascade and the cyclic adenosine 3,5-monophosphate pathway. Based on the findings, the team in question highlighted catechin as a potential antifungal candidate in clinical therapy for the management and prevention of candidosis [156][157]. Similarly, flavonols (myricetin, kaempferol, fisetin, quercetin, 3-hydroxy flavone, and 3,7-dihydroxyflavone), a flavone (luteolin), a flavanone (naringenin), and isoflavones (genistein, biochanin A) inhibit filamentous fungus Cochliobolus lunatus through the inhibition of nucleic acid synthesis [158]. Apigenin is a well-known flavone found in a wide variety of plants and herbs that interferes with the translational activity of fungal foot-and-mouth disease driven by the internal ribosome entry site, and was thus identified as a potential drug for foot-and-mouth disease infection [159]. Carvacrol, a chalcone extracted from Lavandula multifida L. that inhibits the nucleic acid synthesis and disrupts the cellular cytoplasmic membrane, eventually causes apoptosis in various candida species [160]. Gallic acid extracted from Paeonia rockii inhibits the protein synthesis of C. albicans, which has been shown to be involved in a decreasing number of hyphal cells and germ tubes with a MIC of 30 mg/mL [161]. Similarly, gallotannin obtained from Syzygium cordatum inhibits RNA synthesis and possess antifungal activity against C. albicans with an MIC value of 0.195 mg/mL [43].
3.7. Synergistic Action between Flavonoids and Antifungals
The combination of natural products with antifungal drugs is recognized as an effective strategy to fight invasive fungal infections and microbial resistance [162]. This combination is often beneficial and effective for both the rate and degree of microbial killing [163]. Generally, each drug has a diverse mechanism of action, and two drugs may play on diverse targets, resulting in multi-targeting. Based on the multi-targeting strategy, the progress of drug resistance can be reduced [164][165]. Toxicity and intolerance of the drug can also be evaded with the aid of two or more collective drug treatments. Several in vitro studies have shown a reduced inhibitory concentration of natural products with antifungal drugs [166][167][168]. For instance, bioactive compounds help elevate the intracellular concentration of related antifungals by potentiating their action, inhibiting the efflux pumps, and inhibiting the morphogenesis of drug-resistant C. albicans [167][169].
Studies have exhibited that Brazilian Red Propolis and Acca sellowiana produce in vitro synergistic activity with fluconazole against resistant fungal isolates of C. parapsilosis and C. glabrata [166][170]. Propolis offered action on the cell membrane, permitting fluconazole penetration into the cells [166][171]. The synergistic effect accelerates between the extracts of Uncaria tomentosa and fluconazole against Candida non-albicans, and quite likely this effect is connected to teamwork events happening outside the cell membrane [167]. For antifungal therapeutic approaches, a combination of antifungals with the host’s immune system is more essential [61][166][168]. This combination may trigger the healing of lesions and control most of the symptoms connected to fungal infections [168][172]. Hence, the phytotherapy adjuvant is the main healer for fungal infections exclusively for pharmaceutical companies.
Curcumin, when combined with fluconazole, miconazole, ketoconazole, nystatin, and amphotericin B in vitro, results in synergistic interaction against C. albicans [146]. Curcumin elevates the level of ROS and regulation of expression of numerous genes related to fungal oxidative stress, including superoxide dismutase, catalase, and oxydoreductase [144]. Chalcones are naturally occurring flavonoids that have been synthesized by aldol condensation, which possess significant antifungal properties when combined with fluconazole and resistant strains of C. albicans. Chalcones are the main inhibitors of the efflux pump, which in combination with fluconazole decrease the ability of cells to efflux out the drugs [173][174]. Osthole is a natural methylated derivative of coumarin isolated from Candida fructus, which has been extensively used for the treatment of supportive dermatitis and vaginitis in China. It is synergistically combined with fluconazole and possesses significant antifungal effects through the generation of ROS [175]. Similarly, eugenol-tosylate, a semi-synthetic analog of eugenol, has a synergistic interaction with fluconazole that exhibits antifungal activity against fluconazole-resistant C. albicans which occurs through the inhibiting of ergosterol biosynthesis [176][177]. Glabridin exhibits a synergistic combination with fluconazole against resistant strains of C. albicans, causing cell wall alteration [178]. Likewise, quercetin’s synergistic combination with fluconazole inhibits C. albicans biofilm, which is isolated from vulvovaginal candidiasis patients. These drugs combined, have the ability to avert the adhesion of cell-cell communication and disturb the expression of genes accountable for biofilm formation [179].
4. Conclusions
The elevation of fungal infections is alarming. They lead to high levels of morbidity and mortality globally. Emerging new fungal species and the incidence of elevated drug resistance for fungal diseases continues to rise. The scenario of the existing antifungal agents and their complications is quite critical. There are limitations manifest by antifungal agents: the lower fungistatic ability, high toxicity, and kidney failure. Hence, it is vital to search novel agents as alternative therapies that are potentially active against most fungal diseases. Medicinal plants containing flavonoids are recognized as safe and endowed with numerous biological functions. Various flavonoids have been extracted and investigated in association with their anti-fungal activities and can be promising, efficient, and cost-effective agents for the inhibition of fungal infections. They often inhibit fungal growth in various underlying mechanisms by enhancing the disruption of the plasma membrane and mitochondrial dysfunction; and inhibiting cell wall formation, cell division, protein synthesis, and the efflux-mediated pumping system. These flavonoids are capable and efficient in synergetic combination therapy with conventional drugs, which can be more appropriate and supportive for finding novel drug therapies against fungal pathogens.
References
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. Journal of Fungi 2017, 3, 57, doi:10.3390/jof3040057.
- Mickymaray, S.; Alfaiz, F.A.; Paramasivam, A. Efficacy and Mechanisms of Flavonoids against the Emerging Opportunistic Nontuberculous Mycobacteria. Antibiotics (Basel, Switzerland) 2020, 9, 450, doi:10.3390/antibiotics9080450.
- Lu, M.; Li, T.; Wan, J.; Li, X.; Yuan, L.; Sun, S. Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. International Journal of Antimicrobial Agents 2017, 49, 125-136, doi:10.1016/j.ijantimicag.2016.10.021.
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii — A first report from the Kingdom of Saudi Arabia. Journal of Infection and Public Health 2018, 11, 812-816, doi:10.1016/j.jiph.2018.05.011.
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. International Journal of Molecular Sciences 2018, 19, 3720, doi:10.3390/ijms19123720.
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy. Cancers 2019, 11, 1592, doi:10.3390/cancers11101592.
- Sanglard, D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Frontiers in Medicine 2016, 3, doi:10.3389/fmed.2016.00011.
- Lu, S.; Zhang, J.; Chen, X.; Mickymaray, S.; Liu, Z. Limonin: A triterpenoid exerts protective effect during lipopolysaccharide stimulated inflammation in BV2 microglial cells. Pharmacognosy Magazine 2020, 16, 859-864, doi:10.4103/pm.pm_304_19.
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Scientific Reports 2019, 9, doi:10.1038/s41598-019-52525-7.
- Vinodhini R, M.K., Al Aboody MS, Suresh, M. Prevalence And Antifungal Susceptibility Pattern of Candida Dubliniensis Isolated From Urine Samples. Int J Recent Sci Res 2016, 7, 13474-13480.
- Devi AC, D.D., Suresh M, Thajuddin N. Diagnostic value of real time PCR and associated bacterial and fungal infections in female genital tuberculosis. Biomed Pharmacol J 2015, 3, 73-79.
- Mickymaray, S., Al Aboody, M.S., Rath, P.K., Annamalai, P., Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac J Trop Biomed 2016, 6, 185-191.
- Suresh, M.; Alfonisan, M.; Alturaiki, W.; Al Aboody, M.S.; Alfaiz, F.A.; Premanathan, M.; Vijayakumar, R.; Umamagheswari, K.; Ghamdi, S.A.; Alsagaby, S.A. Investigations of Bioactivity of Acalypha indica (L.), Centella asiatica (L.) and Croton bonplandianus (Baill) against Multidrug Resistant Bacteria and Cancer Cells. Journal of Herbal Medicine 2020, 100359, doi:https://doi.org/10.1016/j.hermed.2020.100359.
- Ng, K.P.; Kuan, C.S.; Kaur, H.; Na, S.L.; Atiya, N.; Velayuthan, R.D. Candidaspecies epidemiology 2000-2013: a laboratory-based report. Tropical Medicine & International Health 2015, 20, 1447-1453, doi:10.1111/tmi.12577.
- Wang, Y. Looking intoCandida albicansinfection, host response, and antifungal strategies. Virulence 2015, 6, 307-308, doi:10.1080/21505594.2014.1000752.
- Polke, M.; Hube, B.; Jacobsen, I.D. Candida Survival Strategies. Advances in Applied Microbiology 2015, 139-235, doi:10.1016/bs.aambs.2014.12.002.
- Alsagaby, S.A.; Vijayakumar, R.; Premanathan, M.; Mickymaray, S.; Alturaiki, W.; Al-Baradie, R.S.; AlGhamdi, S.; Aziz, M.A.; Alhumaydhi, F.A.; Alzahrani, F.A.; et al. Transcriptomics-Based Characterization of the Toxicity of ZnO Nanoparticles Against Chronic Myeloid Leukemia Cells. Int J Nanomedicine 2020, 15, 7901-7921, doi:10.2147/ijn.S261636.
- Hurtado, J.C.; Castillo, P.; Fernandes, F.; Navarro, M.; Lovane, L.; Casas, I.; Quintó, L.; Marco, F.; Jordao, D.; Ismail, M.R.; et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Scientific Reports 2019, 9, doi:10.1038/s41598-019-43941-w.
- Molina-Leyva, A.; Ruiz-Carrascosa, J.C.; Leyva-Garcia, A.; Husein-Elahmed, H. Cutaneous Cryptococcus laurentii infection in an immunocompetent child. International Journal of Infectious Diseases 2013, 17, e1232-e1233, doi:10.1016/j.ijid.2013.04.017.
- Smith, N.; Sehring, M.; Chambers, J.; Patel, P. Perspectives on non-neoformanscryptococcal opportunistic infections. Journal of Community Hospital Internal Medicine Perspectives 2017, 7, 214-217, doi:10.1080/20009666.2017.1350087.
- Calista, F.; Tomei, F.; Assalone, P.; Traficante, D.; Di Pilla, G.; Pepe, C.; Di Lullo, L. Cryptococcus laurentii Diarrhea in a Neoplastic Patient. Case Rep Oncol Med 2015, 2015, 216458, doi:10.1155/2015/216458.
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. The Lancet Infectious Diseases 2017, 17, 873-881, doi:10.1016/s1473-3099(17)30243-8.
- Kannaiyan, M.; Meseret Abebe, G.; Kanimozhi, C.; Thambidurai, P.; Ashokapuram Selvam, S.; Vinodhini, R.; Suresh, M. PREVALENCE OF EXTENDED-SPECTRUM BETA-LACTAMASE PRODUCING ENTEROBACTERIACEAE MEMBERS ISOLATED FROM CLINICALLY SUSPECTED PATIENTS. Asian Journal of Pharmaceutical and Clinical Research 2018, 11, 364, doi:10.22159/ajpcr.2018.v11i5.19363.
- Sinaga, M., Ganesan, K., Kumar, Nair, S.K.P., Gani, S.B. Preliminary Phytochemical Analysis and In Vitro Antibacterial Activity of Bark and Seeds of Ethiopian Neem (Azadirachta Indica A. Juss). World J Pharmacy Pharma Sci. 2016, 5, 1714-1723, doi:10.20959/wjpps20164-6479.
- Ito, J.; Kriengkauykiat; Dadwal, S.; Kriengkauykiat. Epidemiology and treatment approaches in management of invasive fungal infections. Clinical Epidemiology 2011, 175, doi:10.2147/clep.s12502.
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harbor Perspectives in Medicine 2014, 4, a019703-a019703, doi:10.1101/cshperspect.a019703.
- Banu, G.S., Kumar, G. In-vitro antibacterial activity of flower extracts of Woodfordia fruticosa Kurz. . Int. J. Curr. Res. Chem. Pharma. Sci. 2014, 1, 127-130.
- Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032, doi:10.3390/molecules23113032.
- Jing, W.; Feng, L.; Wang, B.; Zhang, W.; Xu, K.; Al Aboody, M.S.; Mickymaray, S.; Peng, K. Polymer-ceramic fiber nanocomposite coatings on titanium metal implant devices for diseased bone tissue regeneration. Journal of Science: Advanced Materials and Devices 2021, doi:https://doi.org/10.1016/j.jsamd.2021.04.001.
- Mickymaray, S.; Al Aboody, M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina 2019, 55, 289, doi:10.3390/medicina55060289.
- Mickymaray. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662, doi:10.3390/biom9110662.
- Xu, B.; Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioactive Compounds in Health and Disease 2020, 3, 15, doi:10.31989/bchd.v3i2.687.
- Selvakumar, V.; Kannan, K.; Panneerselvam, A.; Suresh, M.; Nooruddin, T.; Pal, K.; Elkodous, M.A.; Nada, H.G.; El-Bastawisy, H.S.; Tolba, M.M.; et al. Molecular identification of extended spectrum β-lactamases (ESBLs)-producing strains in clinical specimens from Tiruchirappalli, India. Applied Nanoscience 2021, doi:10.1007/s13204-021-01886-5.
- Al-Hosary, A.A.; ElSify, A.; Salama, A.A.; Nayel, M.; Elkhtam, A.; Elmajdoub, L.O.; Rizk, M.A.; Hawash, M.M.; Al-Wabel, M.A.; Almuzaini, A.M.; et al. Phylogenetic study of Theileria ovis and Theileria lestoquardi in sheep from Egypt: Molecular evidence and genetic characterization. Vet World 2021, 14, 634-639, doi:10.14202/vetworld.2021.634-639.
- Chen, H.; Feng, X.; Gao, L.; Mickymaray, S.; Paramasivam, A.; Abdulaziz Alfaiz, F.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Aziz Ibrahim, I.A. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif Cells Nanomed Biotechnol 2021, 49, 240-249, doi:10.1080/21691401.2021.1890101.
- Shi, Q.; Anishiya Chella Daisy, E.R.; GeqiangYang; Zhang, J.; Mickymaray, S.; Alfaiz, F.; Paramasivam, A.; Rajan, M. Multifeatured guar gum armed drug delivery system for the delivery of ofloxacin drug to treat ophthalmic dieases. Arabian Journal of Chemistry 2021, 14, 103118, doi:https://doi.org/10.1016/j.arabjc.2021.103118.
- Mickymaray, S.; Alfaiz, F.A.; Paramasivam, A.; Veeraraghavan, V.P.; Periadurai, N.D.; Surapaneni, K.M.; Niu, G. Rhaponticin suppresses osteosarcoma through the inhibition of PI3K-Akt-mTOR pathway. Saudi Journal of Biological Sciences 2021, doi:https://doi.org/10.1016/j.sjbs.2021.05.006.
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics (Basel) 2020, 9, doi:10.3390/antibiotics9020045.
- Edziri, H.; Mastouri, M.; Mahjoub, M.A.; Mighri, Z.; Mahjoub, A.; Verschaeve, L. Antibacterial, Antifungal and Cytotoxic Activities of Two Flavonoids from Retama raetam Flowers. Molecules 2012, 17, 7284-7293, doi:10.3390/molecules17067284.
- Sohn, H.-Y. Fungicidal Effect of Prenylated Flavonol, Papyriflavonol A, Isolated from Broussonetia papyrifera (L.) Vent. Against Candida albicans. Journal of Microbiology and Biotechnology 2010, 20, 1397-1402, doi:10.4014/jmb.1007.07026.
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiological Research 2010, 165, 496-504, doi:10.1016/j.micres.2009.09.002.
- Wächter, G.A.; Hoffmann, J.J.; Furbacher, T.; Blake, M.E.; Timmermann, B.N. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry 1999, 52, 1469-1471, doi:10.1016/s0031-9422(99)00221-6.
- Mulaudzi, R.B.; Ndhlala, A.R.; Kulkarni, M.G.; Van Staden, J. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. Journal of Ethnopharmacology 2012, 143, 185-193, doi:10.1016/j.jep.2012.06.022.
- Herrera, C.L.; Alvear, M.; Barrientos, L.; Montenegro, G.; Salazar, L.A. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Ciencia e investigación agraria 2010, 37, doi:10.4067/s0718-16202010000100007.
- Serpa, R.; Franca, E.J.G.; Furlaneto-Maia, L.; Andrade, C.G.T.J.; Diniz, A.; Furlaneto, M.C. In vitro antifungal activity of the flavonoid baicalein against Candida species. Journal of Medical Microbiology 2012, 61, 1704-1708, doi:10.1099/jmm.0.047852-0.
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural Products: An Alternative to Conventional Therapy for Dermatophytosis? Mycopathologia 2016, 182, 143-167, doi:10.1007/s11046-016-0081-9.
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Critical Reviews in Food Science and Nutrition 2017, 58, 1165-1229, doi:10.1080/10408398.2016.1244154.
- Ganesan, K.; Nair, S.K.P.; Gulilat, H.; Letha, N.; Gani, S.B. Preliminary phytochemical screening and in vitro antioxidant activity of Datura stramonium L. collected from Jimma, South West Ethiopia. Int J Pharm Bio Sci 2016, 7, 261-266.
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. International Journal of Molecular Sciences 2017, 18, 2331, doi:10.3390/ijms18112331.
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. International Journal of Molecular Sciences 2017, 18, 2390, doi:10.3390/ijms18112390.
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Annals of the New York Academy of Sciences 2017, 1401, 102-113, doi:10.1111/nyas.13446.
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean ( Vigna radiata ). Food Science and Human Wellness 2018, 7, 11-33, doi:10.1016/j.fshw.2017.11.002.
- Correia, A.F.; Silveira, D.; Fonseca-Bazzo, Y.M.; Magalhães, P.O.; Fagg, C.W.; da Silva, E.C.; Gomes, S.M.; Gandolfi, L.; Pratesi, R.; de Medeiros Nóbrega, Y.K. Activity of crude extracts from Brazilian cerrado plants against clinically relevant Candida species. BMC Complementary and Alternative Medicine 2016, 16, doi:10.1186/s12906-016-1164-3.
- Yamaguchi, M.U.; Garcia, F.P.; Cortez, D.A.G.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorifera (Lauraceae). Antonie van Leeuwenhoek 2010, 99, 507-514, doi:10.1007/s10482-010-9516-3.
- Klewicka, E.; Sójka, M.; Klewicki, R.; Kołodziejczyk, K.; Lipińska, L.; Nowak, A. Ellagitannins from Raspberry (Rubus idaeus L.) Fruit as Natural Inhibitors of Geotrichum candidum. Molecules 2016, 21, 908, doi:10.3390/molecules21070908.
- dos Santos, C.; Vargas, Á.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids and Surfaces B: Biointerfaces 2017, 151, 26-33, doi:10.1016/j.colsurfb.2016.11.041.
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents 2005, 26, 343-356, doi:10.1016/j.ijantimicag.2005.09.002.
- Tapas, A.R.; Sakarkar, D.M.; Kakde, R.B. Flavonoids as Nutraceuticals: A Review. Tropical Journal of Pharmaceutical Research 2008, 7, doi:10.4314/tjpr.v7i3.14693.
- Taleb-Contini, S.H.; Salvador, M.J.; Watanabe, E.; Ito, I.Y.; Oliveira, D.C.R.d. Antimicrobial activity of flavonoids and steroids isolated from two Chromolaena species. Revista Brasileira de Ciências Farmacêuticas 2003, 39, 403-408, doi:10.1590/s1516-93322003000400007.
- Li, K.; Xing, S.; Wang, M.; Peng, Y.; Dong, Y.; Li, X. Anticomplement and Antimicrobial Activities of Flavonoids from Entada phaseoloides. Natural Product Communications 2012, 7, 1934578X1200700, doi:10.1177/1934578x1200700715.
- Ahmadi, F.; Sadeghi, S.; Modarresi, M.; Abiri, R.; Mikaeli, A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food and Chemical Toxicology 2010, 48, 1137-1144, doi:10.1016/j.fct.2010.01.028.
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.; de Torres, N. Activity of Polyphenolic Compounds against Candida glabrata. Molecules 2015, 20, 17903-17912, doi:10.3390/molecules201017903.
- Montagner, C.; de Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.A.; Smânia Jr, A. Antifungal Activity of Coumarins. Zeitschrift für Naturforschung C 2008, 63, 21-28, doi:10.1515/znc-2008-1-205.
- Navarro-García, V.M.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 2011, 54, e569-e571, doi:10.1111/j.1439-0507.2010.01993.x.
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Phenylpropanoids of Plant Origin as Inhibitors of Biofilm Formation by Candida albicans. Journal of Microbiology and Biotechnology 2014, 24, 1216-1225, doi:10.4014/jmb.1402.02056.
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chemistry 2011, 124, 1411-1415, doi:10.1016/j.foodchem.2010.07.100.
- Mendoza, L.; Yañez, K.; Vivanco, M.; Melo, R.; Cotoras, M. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Industrial Crops and Products 2013, 43, 360-364, doi:10.1016/j.indcrop.2012.07.048.
- Han, Y. Synergic Anticandidal Effect of Epigallocatechin-O-gallate Combined with Amphotericin B in a Murine Model of Disseminated Candidiasis and Its Anticandidal Mechanism. Biological & Pharmaceutical Bulletin 2007, 30, 1693-1696, doi:10.1248/bpb.30.1693.
- Han, Y. Synergic effect of grape seed extract with amphotericin B against disseminated candidiasis due to Candida albicans. Phytomedicine 2007, 14, 733-738, doi:10.1016/j.phymed.2007.08.004.
- da Silva, C.R.; de Andrade Neto, J.B.; de Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; de Andrade, G.M.; Lima, I.S.P.; et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrobial Agents and Chemotherapy 2013, 58, 1468-1478, doi:10.1128/aac.00651-13.
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. Journal of Applied Microbiology 2007, 103, 2056-2064, doi:10.1111/j.1365-2672.2007.03456.x.
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chemistry 2011, 126, 1749-1758, doi:10.1016/j.foodchem.2010.12.075.
- Abat, J.K.; Kumar, S.; Mohanty, A. Ethnomedicinal, Phytochemical and Ethnopharmacological Aspects of Four Medicinal Plants of Malvaceae Used in Indian Traditional Medicines: A Review. Medicines 2017, 4, 75, doi:10.3390/medicines4040075.
- Bitencourt, T.A.; Macedo, C.; Franco, M.E.; Rocha, M.C.; Moreli, I.S.; Cantelli, B.A.M.; Sanches, P.R.; Beleboni, R.O.; Malavazi, I.; Passos, G.A.; et al. Trans-chalcone activity against Trichophyton rubrum relies on an interplay between signaling pathways related to cell wall integrity and fatty acid metabolism. BMC Genomics 2019, 20, doi:10.1186/s12864-019-5792-0.
- Terças, A.G.; Monteiro, A.d.S.; Moffa, E.B.; Santos, J.R.A.d.; Sousa, E.M.d.; Pinto, A.R.B.; Costa, P.C.d.S.; Borges, A.C.R.; Torres, L.M.B.; Barros Filho, A.K.D.; et al. Phytochemical Characterization of Terminalia catappa Linn. Extracts and Their antifungal Activities against Candida spp. Frontiers in Microbiology 2017, 8, doi:10.3389/fmicb.2017.00595.
- Bottari, N.B.; Lopes, L.Q.S.; Pizzuti, K.; Filippi dos Santos Alves, C.; Corrêa, M.S.; Bolzan, L.P.; Zago, A.; de Almeida Vaucher, R.; Boligon, A.A.; Giongo, J.L.; et al. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microbial Pathogenesis 2017, 104, 190-195, doi:10.1016/j.micpath.2017.01.037.
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, Á.C.B.; Koga-Ito, C.Y. Effects of Acetone Fraction From Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors. Frontiers in Microbiology 2018, 9, doi:10.3389/fmicb.2018.00647.
- Reddy, M.; Gupta, S.; Jacob, M.; Khan, S.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Medica 2007, 73, 461-467, doi:10.1055/s-2007-967167.
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. International Journal of Antimicrobial Agents 2014, 44, 269-273, doi:10.1016/j.ijantimicag.2014.05.017.
- da Silva, D.L.; Magalhães, T.F.F.; dos Santos, J.R.A.; de Paula, T.P.; Modolo, L.V.; de Fátima, A.; Buzanello Martins, C.V.; Santos, D.A.; de Resende-Stoianoff, M.A. Curcumin enhances the activity of fluconazole againstCryptococcus gattii-induced cryptococcosis infection in mice. Journal of Applied Microbiology 2015, 120, 41-48, doi:10.1111/jam.12966.
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The Anti-Adhesive Effect of Curcumin on Candida albicans Biofilms on Denture Materials. Frontiers in Microbiology 2017, 8, doi:10.3389/fmicb.2017.00659.
- Paul, S.; Mohanram, K.; Kannan, I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. AYU (An international quarterly journal of research in Ayurveda) 2018, 39, 182, doi:10.4103/ayu.ayu_24_18.
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clinical Microbiology Reviews 1999, 12, 501-517, doi:10.1128/cmr.12.4.501.
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. Fungi 2005, 1-34, doi:10.1002/0470015330.ch1.
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. Journal of Medical Microbiology 2009, 58, 1454-1462, doi:10.1099/jmm.0.010538-0.
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: induction, repair and significance. Mutation Research/Reviews in Mutation Research 2004, 567, 1-61, doi:10.1016/j.mrrev.2003.11.001.
- Wong-ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Peter Tieleman, D.; Monticelli, L. Effect of Lipid Peroxidation on the Properties of Lipid Bilayers: A Molecular Dynamics Study. Biophysical Journal 2007, 93, 4225-4236, doi:10.1529/biophysj.107.112565.
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132-145, doi:10.1016/j.biochi.2016.05.013.
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Induction of oxidative stress as a possible mechanism of the antifungal action of three phenylpropanoids. FEMS Yeast Research 2010, 11, 114-122, doi:10.1111/j.1567-1364.2010.00697.x.
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Félix, E.; Carvajal-Millán, E.; González-Córdova, A.F.; Vallejo-Galland, B.; Torres-Llanez, M.J.; Sánchez-Escalante, A. Antioxidant and Antimicrobial Activity of Commercial Propolis Extract in Beef Patties. Journal of Food Science 2014, 79, C1499-C1504, doi:10.1111/1750-3841.12533.
- Ganesan; Xu. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556, doi:10.3390/molecules24142556.
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int J Mol Sci 2020, 21, doi:10.3390/ijms21113976.
- Nair, S.K.P.; Ganesan, K.; Azalewor, H.; Letha, N.; Gani, S. Preliminary Phytochemical Screening and In vitro Antioxidant Activity of Ethiopian Indigenous Medicinal Plants, Ocimum lamiifolium Hochst. ex Benth and Ocimum basilicum L. International Journal of Pharmaceutical Sciences and Drug Research 2016, 8, doi:10.25004/ijpsdr.2016.080105.
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): phytochemistry and pharmacological properties. Phytochemistry Reviews 2017, 16, 461-478, doi:10.1007/s11101-017-9493-5.
- Tadesse, S.; Ganesan, K.; Nair, S.; Letha, N.; Gani, S. Preliminary phytochemical investigation of Different Solvent Extracts of Centella asiatica L. (Family: Apiaceae)- an Ethiopian weed. Int J Pharma Chem Biol Sci. 2016, 6, 97-102.
- Tadesse, S.; Kumar, G.; Nair, S.; Letha, N.; Gani, S. Preliminary phytochemical screening of Euphorbia Cotinifolia L. (Family: Euphorbiacae) by using different solvent extracts. World J Pharmacy Pharma Sci. 2016, 5, 1176-1183.
- Lee, H.; Woo, E.-R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Research 2018, 18, doi:10.1093/femsyr/foy003.
- Cantelli, B.A.M.; Bitencourt, T.A.; Komoto, T.T.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Caffeic acid and licochalcone A interfere with the glyoxylate cycle of Trichophyton rubrum. Biomedicine & Pharmacotherapy 2017, 96, 1389-1394, doi:10.1016/j.biopha.2017.11.051.
- Perez, C.; Tiraboschi, I.N.; Ortega, M.G.; Agnese, A.M.; Cabrera, J.L. Further Antimicrobial Studies of 2'4'-dihidroxy-5'-(1?-dimethylallyl)-6-prenylpinocembrin from Dalea elegans. Pharmaceutical Biology 2003, 41, 171-174, doi:10.1076/phbi.41.3.171.15090.
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975-980, doi:10.1016/j.phymed.2015.07.003.
- Ganesan, K.; Sukalingam, K.; Xu, B. Solanum trilobatum L. Ameliorate Thioacetamide-Induced Oxidative Stress and Hepatic Damage in Albino Rats. Antioxidants (Basel) 2017, 6, doi:10.3390/antiox6030068.
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int J Mol Sci 2017, 19, doi:10.3390/ijms19010013.
- Ganesan, K.; Xu, B. Ethnobotanical studies on folkloric medicinal plants in Nainamalai, Namakkal District, Tamil Nadu, India. Trends in Phytochemical Research 2017, 1, 153-168.
- Ganesan, K.; Xu, B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules 2018, 23, 2880, doi:10.3390/molecules23112880.
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. International Journal of Medicinal Mushrooms 2019, 21, 237-251, doi:10.1615/intjmedmushrooms.2019030079.
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chemico-Biological Interactions 2019, 303, 62-69, doi:10.1016/j.cbi.2019.02.017.
- Kumar, G.; Gani, S.; Murugesan, A.G.; Pandian, M.R. Preliminary Toxicity and Phytochemical Studies of Aqueous Bark Extract of Helicteres isora L. Int J Pharmacol 2007, 3, 96-100, doi: 10.3923/ijp.2007.96.100.
- Tsang, P.W.-K.; Chau, K.-Y.; Yang, H.-P. Baicalein exhibits inhibitory effect on the energy-dependent efflux pump activity in non-albicans Candidafungi. Journal of Chemotherapy 2014, 27, 61-62, doi:10.1179/1973947814y.0000000177.
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Antifungal Activity of Baicalein Against Candida krusei Does Not Involve Apoptosis. Mycopathologia 2010, 170, 391-396, doi:10.1007/s11046-010-9341-2.
- Dai, B.D.; Cao, Y.Y.; Huang, S.; Xu, Y.G.; Gao, P.H.; Wang, Y.; Jiang, Y.Y. Baicalein induces programmed cell death in Candida albicans. J Microbiol Biotechnol 2009, 19, 803-809.
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Scientific Reports 2019, 9, doi:10.1038/s41598-019-38916-w.
- da Costa, M.P.; Bozinis, M.C.V.; Andrade, W.M.; Costa, C.R.; da Silva, A.L.; Alves de Oliveira, C.M.; Kato, L.; Fernandes, O.d.F.L.; Souza, L.K.H.; Silva, M.d.R.R. Antifungal and cytotoxicity activities of the fresh xylem sap of Hymenaea courbaril L. and its major constituent fisetin. BMC Complementary and Alternative Medicine 2014, 14, doi:10.1186/1472-6882-14-245.
- Reis, M.P.C.; Carvalho, C.R.C.; Andrade, F.A.; Fernandes, O.F.L.; Arruda, W.; Silva, M.R.R. Fisetin as a promising antifungal agent againstCryptocococcus neoformansspecies complex. Journal of Applied Microbiology 2016, 121, 373-379, doi:10.1111/jam.13155.
- Li, X.-C.; Joshi, A.S.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel, S.E.; et al. Fatty Acid Synthase Inhibitors from Plants: Isolation, Structure Elucidation, and SAR Studies. Journal of Natural Products 2002, 65, 1909-1914, doi:10.1021/np020289t.
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complementary and Alternative Medicine 2013, 13, doi:10.1186/1472-6882-13-229.
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.-R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. Biochimica et Biophysica Acta (BBA) - Biomembranes 2015, 1848, 695-701, doi:10.1016/j.bbamem.2014.11.019.
- Lagrouh, F.; Dakka, N.; Bakri, Y. The antifungal activity of Moroccan plants and the mechanism of action of secondary metabolites from plants. Journal de Mycologie Médicale 2017, 27, 303-311, doi:10.1016/j.mycmed.2017.04.008.
- Liu, W.; Li, L.P.; Zhang, J.D.; Li, Q.; Shen, H.; Chen, S.M.; He, L.J.; Yan, L.; Xu, G.T.; An, M.M.; et al. Synergistic Antifungal Effect of Glabridin and Fluconazole. PLoS ONE 2014, 9, e103442, doi:10.1371/journal.pone.0103442.
- Moazeni, M.; Hedayati, M.T.; Nabili, M.; Mousavi, S.J.; Abdollahi Gohar, A.; Gholami, S. Glabridin triggers over-expression of MCA1 and NUC1 genes in Candida glabrata : Is it an apoptosis inducer? Journal de Mycologie Médicale 2017, 27, 369-375, doi:10.1016/j.mycmed.2017.05.002.
- Sangalli-Leite, F.; Scorzoni, L.; Alves de Paula e Silva, A.C.; da Silva, J.d.F.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; Moraes da Silva, R.; Regasini, L.O.; Siqueira da Silva, D.H.; et al. Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. International Journal of Antimicrobial Agents 2016, 48, 504-511, doi:10.1016/j.ijantimicag.2016.07.025.
- Yun, D.G.; Lee, D.G. Silymarin exerts antifungal effects via membrane-targeted mode of action by increasing permeability and inducing oxidative stress. Biochimica et Biophysica Acta (BBA) - Biomembranes 2017, 1859, 467-474, doi:10.1016/j.bbamem.2017.01.009.
- Cao, Y.; Dai, B.; Wang, Y.; Huang, S.; Xu, Y.; Cao, Y.; Gao, P.; Zhu, Z.; Jiang, Y. In vitro activity of baicalein against Candida albicans biofilms. International Journal of Antimicrobial Agents 2008, 32, 73-77, doi:10.1016/j.ijantimicag.2008.01.026.
- Oliveira, M.R.d.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria—A story of life and death. Pharmacological Research 2016, 104, 70-85, doi:10.1016/j.phrs.2015.12.027.
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Brazilian Journal of Biology 2016, 76, 1029-1034, doi:10.1590/1519-6984.07415.
- Gibellini, L.; Bianchini, E.; De Biasi, S.; Nasi, M.; Cossarizza, A.; Pinti, M. Natural Compounds Modulating Mitochondrial Functions. Evidence-Based Complementary and Alternative Medicine 2015, 2015, 1-13, doi:10.1155/2015/527209.
- Guntuku, L.; Naidu, V.G.M.; Ganesh Yerra, V. Mitochondrial Dysfunction in Gliomas: Pharmacotherapeutic Potential of Natural Compounds. Current Neuropharmacology 2016, 14, 567-583, doi:10.2174/1570159x14666160121115641.
- Canonico, B.; Candiracci, M.; Citterio, B.; Curci, R.; Squarzoni, S.; Mazzoni, A.; Papa, S.; Piatti, E. Honey flavonoids inhibit Candida albicans morphogenesis by affecting DNA behavior and mitochondrial function. Future Microbiology 2014, 9, 445-456, doi:10.2217/fmb.14.17.
- Ning, Y.; Ling, J.; Wu, C.D. Synergistic effects of tea catechin epigallocatechin gallate and antimycotics against oral Candida species. Archives of Oral Biology 2015, 60, 1565-1570, doi:10.1016/j.archoralbio.2015.07.001.
- da Costa Cordeiro, B.M.P.; de Lima Santos, N.D.; Ferreira, M.R.A.; de Araújo, L.C.C.; Junior, A.R.C.; da Conceição Santos, A.D.; de Oliveira, A.P.; da Silva, A.G.; da Silva Falcão, E.P.; dos Santos Correia, M.T.; et al. Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damages. BMC Complementary and Alternative Medicine 2018, 18, doi:10.1186/s12906-018-2350-2.
- Reiners, J.J. Suppression of cell cycle progression by flavonoids: dependence on the aryl hydrocarbon receptor. Carcinogenesis 1999, 20, 1561-1566, doi:10.1093/carcin/20.8.1561.
- Manoharan, R.K.; Lee, J.-H.; Kim, Y.-G.; Lee, J. Alizarin and Chrysazin Inhibit Biofilm and Hyphal Formation by Candida albicans. Frontiers in Cellular and Infection Microbiology 2017, 7, doi:10.3389/fcimb.2017.00447.
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLOS ONE 2015, 10, e0117695, doi:10.1371/journal.pone.0117695.
- Han, B.; Chen, J.; Yu, Y.-q.; Cao, Y.-b.; Jiang, Y.-y. Antifungal activity ofRubus chingiiextract combined with fluconazole against fluconazole-resistantCandida albicans. Microbiology and Immunology 2016, 60, 82-92, doi:10.1111/1348-0421.12357.
- Wang, D.; Sun, Q.; Wu, J.; Wang, W.; Yao, G.; Li, T.; Li, X.; Li, L.; Zhang, Y.; Cui, W.; et al. A new Prenylated Flavonoid induces G0/G1 arrest and apoptosis through p38/JNK MAPK pathways in Human Hepatocellular Carcinoma cells. Scientific Reports 2017, 7, doi:10.1038/s41598-017-05955-0.
- Kannaiyan, M.; Saranya, A.; Suresh, M.; Al Aboody, M.; Rath, P.; Kibemo, B. Prevalence of metallo-β-lactamases in Carbapenem Resistant Acinetobacter Baumannii Isolated from tracheal secretions. Int J Recent Sci Res 2017, 8, 15197-15202.
- Al Aboody, M.; Suresh, M.; Vinodhini, R.; Moorthy, K. Prevalence and antifungal susceptibility pattern of Candida dubliniensis isolated from urine samples. Int J Recent Sci Res. 2016, 7, 13474-13480.
- Nachimuthu, R.; Subramani, R.; Maray, S.; Gothandam, K.M.; Sivamangala, K.; Manohar, P.; Bozdogan, B. Characterization of carbapenem-resistant Gram-negative bacteria from Tamil Nadu. Journal of Chemotherapy 2016, 28, 371-374, doi:10.1179/1973947815y.0000000056.
- Kang, K.; Fong, W.-P.; Tsang, P.W.-K. Novel antifungal activity of purpurin againstCandidaspeciesin vitro. Medical Mycology 2010, 48, 904-911, doi:10.3109/13693781003739351.
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artificial Cells, Nanomedicine, and Biotechnology 2019, 47, 1938-1946, doi:10.1080/21691401.2019.1614017.
- Mangoyi, R.; Midiwo, J.; Mukanganyama, S. Isolation and characterization of an antifungal compound 5-hydroxy-7,4’-dimethoxyflavone from Combretum zeyheri. BMC Complementary and Alternative Medicine 2015, 15, doi:10.1186/s12906-015-0934-7.
- Huang, S.; Cao, Y.Y.; Dai, B.D.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In Vitro Synergism of Fluconazole and Baicalein against Clinical Isolates of Candida albicans Resistant to Fluconazole. Biological & Pharmaceutical Bulletin 2008, 31, 2234-2236, doi:10.1248/bpb.31.2234.
- Mickymaray. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257, doi:10.3390/antibiotics8040257.
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS Yeast Research 2015, 15, doi:10.1093/femsyr/fov001.
- Sharma, M.; Manoharlal, R.; Shukla, S.; Puri, N.; Prasad, T.; Ambudkar, S.V.; Prasad, R. Curcumin Modulates Efflux Mediated by Yeast ABC Multidrug Transporters and Is Synergistic with Antifungals. Antimicrobial Agents and Chemotherapy 2009, 53, 3256-3265, doi:10.1128/aac.01497-08.
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pacific Journal of Tropical Biomedicine 2016, 6, 185-191, doi:10.1016/j.apjtb.2015.12.005.
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida albicans. Antimicrobial Agents and Chemotherapy 2011, 55, 4834-4843, doi:10.1128/aac.00344-11.
- Bozdogana, B.; Ramesh, N.; Prasanth, M.; Ramkumar, S.; Suresh, M.; Tamhankar, A.J.; Gothandam, K.M.; Karthikeyan, S. Colistin susceptibility of Gram-Negative clinical isolates from Tamil Nadu, India Asian Biomedicine 2016, 10, , 35-39.
- Yiğit, D.; Yiğit, N.; Mavi, A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Brazilian Journal of Medical and Biological Research 2009, 42, 346-352, doi:10.1590/s0100-879x2009000400006.
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In Vitro and In Vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms. PLOS ONE 2016, 11, e0157188, doi:10.1371/journal.pone.0157188.
- Belofsky, G.; Kolaczkowski, M.; Adams, E.; Schreiber, J.; Eisenberg, V.; Coleman, C.M.; Zou, Y.; Ferreira, D. Fungal ABC Transporter-Associated Activity of Isoflavonoids from the Root Extract ofDalea formosa. Journal of Natural Products 2013, 76, 915-925, doi:10.1021/np4000763.
- Mbaveng, A.T.; Kuete, V.; Ngameni, B.; Beng, V.P.; Ngadjui, B.T.; Meyer, J.J.M.; Lall, N. Antimicrobial activities of the methanol extract and compounds from the twigs of Dorstenia mannii (Moraceae). BMC Complementary and Alternative Medicine 2012, 12, doi:10.1186/1472-6882-12-83.
- Suresh, M.; Nithya, N.; Jayasree, P.R.; Kumar, P.M. DETECTION AND PREVALENCE OF EFFLUX PUMP-MEDIATED DRUG RESISTANCE IN CLINICAL ISOLATES OF MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIA FROM NORTH KERALA, INDIA. Asian Journal of Pharmaceutical and Clinical Research 2016, 9, 324-327.
- Lim, S.S.; Selvaraj, A.; Ng, Z.Y.; Palanisamy, M.; Mickmaray, S.; Cheong, P.C.H.; Lim, R.L.H. Isolation of actinomycetes with antibacterial activity against multi-drug resistant bacteria. Malaysian Journal of Microbiology 2018, doi:10.21161/mjm.110617.
- Moudgal, V.; Sobel, J. Antifungals to treatCandida albicans. Expert Opinion on Pharmacotherapy 2010, 11, 2037-2048, doi:10.1517/14656566.2010.493875.
- Vinodhini, R.; Moorthy, K.; Suresh, M. INCIDENCE AND VIRULENCE TRAITS OF CANDIDA DUBLINIENSIS ISOLATED FROM CLINICALLY SUSPECTED PATIENTS. Asian Journal of Pharmaceutical and Clinical Research 2016, 9, 77, doi:10.22159/ajpcr.2016.v9i6.13567.
- Saito, H.; Tamura, M.; Imai, K.; Ishigami, T.; Ochiai, K. Catechin inhibits Candida albicans dimorphism by disrupting Cek1 phosphorylation and cAMP synthesis. Microbial Pathogenesis 2013, 56, 16-20, doi:10.1016/j.micpath.2013.01.002.
- Mickymaray, S.; Alturaiki, W.; Al-Aboody, M.S.; Mariappan, P.; Rajenderan, V.; Alsagaby, S.A.; Kalyanasundram, U.; Alarfajj, A.A. Anti-bacterial Efficacy of Bacteriocin Produced by Marine Bacillus subtilis Against Clinically Important Extended Spectrum Beta-Lactamase Strains and Methicillin-Resistant Staphylococcus aureus. International Journal of Medical Research and Health Sciences 2018, 7, 75-83.
- Cassetta, A.; Stojan, J.; Krastanova, I.; Kristan, K.; Brunskole Švegelj, M.; Lamba, D.; Lanišnik Rižner, T. Structural basis for inhibition of 17β-hydroxysteroid dehydrogenases by phytoestrogens: The case of fungal 17β-HSDcl. The Journal of Steroid Biochemistry and Molecular Biology 2017, 171, 80-93, doi:10.1016/j.jsbmb.2017.02.020.
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity. Viruses 2015, 7, 1613-1626, doi:10.3390/v7041613.
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. European Journal of Clinical Microbiology & Infectious Diseases 2011, 31, 1359-1366, doi:10.1007/s10096-011-1450-4.
- Picerno, P.; Mencherini, T.; Sansone, F.; Del Gaudio, P.; Granata, I.; Porta, A.; Aquino, R.P. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. Journal of Ethnopharmacology 2011, 138, 705-712, doi:10.1016/j.jep.2011.09.056.
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination Treatment of Invasive Fungal Infections. Clinical Microbiology Reviews 2005, 18, 163-194, doi:10.1128/cmr.18.1.163-194.2005.
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639-652, doi:10.1016/j.phymed.2008.06.008.
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97-110, doi:10.1016/j.phymed.2008.12.018.
- Vijayakumar, R.; Aboody, M.S.A.; AlFonaisan, M.K.; Turaiki, W.A.; Mickymaray, S.; Mariappan, P.; Alsagaby, S.A.; Sandle, T. Determination of Minimum inhibitory concentrations of Common Biocides to Multidrug-Resistant Gram-negative bacteria. 2016.
- Pippi, B.; Lana, A.J.D.; Moraes, R.C.; Güez, C.M.; Machado, M.; de Oliveira, L.F.S.; Lino von Poser, G.; Fuentefria, A.M. In vitroevaluation of the acquisition of resistance, antifungal activity and synergism of Brazilian red propolis with antifungal drugs onCandidaspp. Journal of Applied Microbiology 2015, 118, 839-850, doi:10.1111/jam.12746.
- Moraes, R.C.; Carvalho, A.R.; Lana, A.J.D.; Kaiser, S.; Pippi, B.; Fuentefria, A.M.; Ortega, G.G. In vitrosynergism of a water insoluble fraction ofUncaria tomentosacombined with fluconazole and terbinafine against resistant non-Candida albicansisolates. Pharmaceutical Biology 2016, 55, 406-415, doi:10.1080/13880209.2016.1242631.
- Danielli, L.J.; Pippi, B.; Soares, K.D.; Duarte, J.A.; Maciel, A.J.; Machado, M.M.; Oliveira, L.F.S.; Bordignon, S.A.L.; Fuentefria, A.M.; Apel, M.A. Chemosensitization of filamentous fungi to antifungal agents using Nectandra Rol. ex Rottb. species essential oils. Industrial Crops and Products 2017, 102, 7-15, doi:10.1016/j.indcrop.2017.03.013.
- Devi, A.C.; Dhanasekaran, D.; Suresh, M.; Thajuddin, N. Diagnostic value of real time PCR and associated bacterial and fungal infections in female genital tuberculosis. Biomed Pharmacol J 2015, 3, 73-79.
- Prabakar, K.; Sivalingam, P.; Mohamed Rabeek, S.I.; Muthuselvam, M.; Devarajan, N.; Arjunan, A.; Karthick, R.; Suresh, M.M.; Wembonyama, J.P. Evaluation of antibacterial efficacy of phyto fabricated silver nanoparticles using Mukia scabrella (Musumusukkai) against drug resistance nosocomial gram negative bacterial pathogens. Colloids and Surfaces B: Biointerfaces 2013, 104, 282-288, doi:10.1016/j.colsurfb.2012.11.041.
- Senthinath, T.; Revathi, P.; Rath, P.K.; Suresh, M.; Vigneshwari, R.S.; Uma, A.; Subramanian, P.T.K.; Moorthy, K.; Thajuddin, N. Antimicrobial resistant pattern of gram negative bacteria to third generation cephalosporins in rural and urban centres of Tamilnadu, India. Int J Biol Technol 2012, 3, 32-38.
- Suresh, M., Rath, P.K., Panneerselvam, A., Dhanasekaran, D., Thajuddin, N. Anti-mycobacterial effect of leaf extract of Centella asiatica. Res. J. Pharmacy Technol. 2010, 3, 872-876.
- Wang, Y.-H.; Dong, H.-H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.-Y.; Jin, Y.-S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorganic & Medicinal Chemistry Letters 2016, 26, 3098-3102, doi:10.1016/j.bmcl.2016.05.013.
- Selvam, P.; Chandramohan, M.; Vivekananthan, S.C.; Sivakumar, D.; Suresh, M.; Rath, P.K. Liver biopsy tissue-real time polymerase chain reaction (RT-PCR) viral load is the only gold standard diagnostic assay in inactive viral hepatitis patients. . Antiviral Res 2010, 86 A44.
- Li, D.-D.; Chai, D.; Huang, X.-W.; Guan, S.-X.; Du, J.; Zhang, H.-Y.; Sun, Y.; Jiang, Y.-Y. PotentIn VitroSynergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrobial Agents and Chemotherapy 2017, 61, doi:10.1128/aac.00436-17.
- Ahmad, A.; Wani, M.Y.; Khan, A.; Manzoor, N.; Molepo, J. Synergistic Interactions of Eugenol-tosylate and Its Congeners with Fluconazole against Candida albicans. PLOS ONE 2015, 10, e0145053, doi:10.1371/journal.pone.0145053.
- Suresh, M., Rath, P.K., Panneerselvam, A., Dhanasekaran, D., Thajuddin, N. Antifungal activity of selected Indian medicinal plant salts. J.Global Pharmacy Technol. 2010, 2, 71-74.
- Fatima, A.; Gupta, V.K.; Luqman, S.; Negi, A.S.; Kumar, J.K.; Shanker, K.; Saikia, D.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P.S. Antifungal activity ofGlycyrrhiza glabraextracts and its active constituent glabridin. Phytotherapy Research 2009, 23, 1190-1193, doi:10.1002/ptr.2726.
- Gao, M.; Wang, H.; Zhu, L. Quercetin Assists Fluconazole to Inhibit Biofilm Formations of Fluconazole-Resistant Candida albicans in In Vitro and In Vivo Antifungal Managements of Vulvovaginal Candidiasis. Physiol. Biochem. 2016, 40, 727–742, doi:10.1159/000453134.




