
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Batista Duharte | + 10071 word(s) | 10071 | 2021-06-04 04:51:27 | | | |
| 2 | Vivi Li | -4867 word(s) | 5204 | 2021-06-07 05:37:35 | | |
Video Upload Options
Antisense oligonucleotides (ASOs) are synthetically prepared short single-stranded deoxynucleotide sequences that have been validated as therapeutic agents and as a valuable tool in molecular driving biology. ASOs can block the expression of specific target genes via complementary hybridization to mRNA. Due to their high specificity and well-known mechanism of action, there has been a growing interest in using them for improving vaccine efficacy. Several studies have shown that ASOs can improve the efficacy of vaccines either by inducing antigen modification such as enhanced expression of immunogenic molecules or by targeting certain components of the host immune system to achieve the desired immune response. However, despite their extended use, some problems such as insufficient stability and low cellular delivery have not been sufficiently resolved to achieve effective and safe ASO-based vaccines.
1. Introduction
1.1. Earlier Uses of Oligonucleotides in Vaccines
1.2. Birth of ASOs
| Chemical Modifications | Characteristics | Mechanisms | Clinical Use | Limitations |
|---|---|---|---|---|
| First Generation | ||||
| Phosphorothioate (PTO), Methylphosphonate (MPO) |
Either a sulfur atom (PTO), or a methyl group (MPO) substitutes the non-bridging oxygen atoms in the phosphodiester bond. | First generation ASOs promote degradation of target mRNA by RNase H enzyme. They also confer higher solubility, resistance to nuclease degradation, antisense activity and longer plasma half-life as compared with phosphodiester oligonucleotides. | PTO is the most widely used modification of ASOs. Fomivirsen, is a PTO-modified ASO, used as local treatment of cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS) [48]. | High affinity for various cellular proteins and components of the innate immune system, such as Toll-like receptors (TLRs), with proinflammatory effects. Commonly reported side effects following systemic administration of PTO ASOs include fever, activated partial thromboplastin time prolongation, thrombocytopenia, and leukopenia. |
| Second Generation | ||||
| ASOs with 2’-O-alkyl modifications of the ribose. Chimeric ‘gapmer’ ASOs |
2’-O-Methyl (2’-OMe) and 2’-O-Methoxyethyl (2’-MOE) are the most widely studied. Chimeric ‘gapmer’ ASOs consist in a central ‘gap’ region containing 10 DNA or PTO DNA monomers, flanked on both 5’ and 3’extremities by alkyl modified nucleotides such as 2′-OM or 2’-MOE. |
The PTO DNA induces RNase H cleavage while 2′-OME or 2′-MOE on both sides (5′- and 3′-directions) confers nuclease-resistance, and they can exert activity by a steric interference of translation process. They are safer than PTO-modified ASOs and exhibit enhanced affinity towards the complementary RNA with better tissue uptake and longer in vivo half-life. |
Mipomersen is used as an adjunct therapy for homozygous familial hypercholesterolemia [49]. Nusinersen was approved for spinal muscular atrophy treatment [50]. Apatorsen is a HSP27 targeting ASO that is being studied in phase II clinical trials in patients with metastatic castration resistant prostate cancer [51] and Untreated Stage IV Non-Squamous-Non-Small-Cell Lung Cancer [52]. |
A subset of 2´-MOE-modified ASOs induced pro-inflammatory cytokines and type I interferons (IFN-α/β) and interaction with innate immune receptors such as TLR9, melanoma-differentiation associated-5 (MDA-5) and IFN-β promoter stimulator-1 (IPS-1). |
| Third Generation | ||||
| Peptide nucleic acid (PNA) | PNA is a synthetic DNA in which the deoxyribose phosphate backbone is replaced by polyamide linkages. | PNA block the protein expression, by steric hindrance, forming sequence-specific duplex with the targeted mRNA. They are biologically stable and have good hybridization properties. | The potential of PNA as drugs in gene therapy has been hampered by the poor intrinsic uptake of PNA by living cells. Current strategies for improving PNA delivery into the cytosolic space and nucleus include microinjection, electroporation, co-transfection with DNA, or conjugation to lipophilic moieties, nanoparticles, cell-penetrating peptides (CPPs), oligo-aspartic acid, or nuclear localization signal (NLS) peptides to enhance cellular internalization | PNA do not activate the RNase H to cleave the target hybridized RNA. PNA have low solubility and cellular uptake. |
| Phosphoramidate morpholino oligomer (PMO) | PMOs are neutral ASOs. The pentose sugar is substituted by a morpholino ring and the inter-nucleotide linkages are phosphoramidate bonds in place of phosphodiester bonds. | The mechanism of PMO is the translational arrest mediated by steric interference of ribosomal assembly. PMO show fewer nonspecific properties and lesser toxicity than PTO. | Eteplirsen was approved for Duchenne muscular dystrophy (DMD) treatment [53]. Other potential applications include the treatment of viral infections, antibiotic-resistant bacterial infections, and cancers [54]. | PMOs exhibit reduced cellular uptake. Conjugation with peptides such as arginine-rich peptide (ARP) can enhance its cellular uptake and antisense efficacy. |
| Locked nucleic acid (LNA) | LNAs are chemically modified nucleotides with a ribose containing a methylene bridge between the 2′-oxygen and the 4′-carbon of the ribose. | LNA modifications improve the affinity of ASO hybridization towards mRNA target, by increase of the DNA/RNA heteroduplexes thermal stability. LNAs avoid nuclease degradation. | Diverse LNAs are currently in clinical trials by several biotechnology firms. | LNA does not activate RNase. LNA nucleotides can be incorporated at the ends of RNA and DNA sequences to form chimeric oligonucleotides resulting in restoration of RNase H-mediated cleavage of mRNA. |
2. ASOs in Vaccines
2.1. Antigen Modification
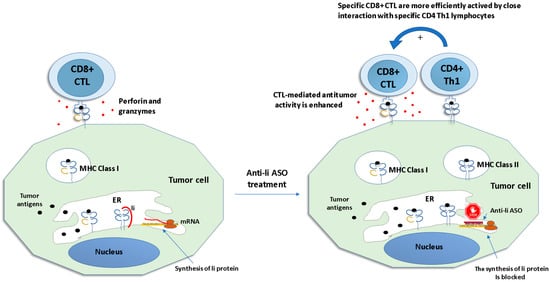
2.2. Targeting Host Immune Mechanisms
3. ASOs as Vaccine Adjuvants in Subunit Vaccines
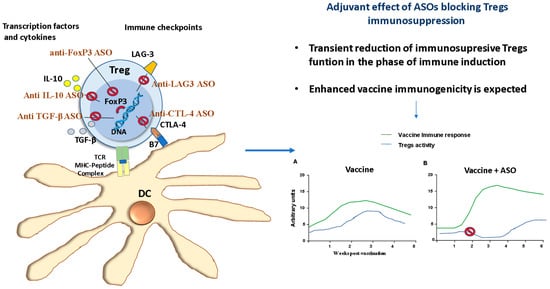
4. Challenges and Opportunities for ASOs Application in Vaccinology
-
Discovery of new suitable genes to improve vaccine protective immunogenicity against specific infectious or tumoral disease using ASOs.
-
Development of bioinformatic tools and in vitro systems for ASOs screening to vaccine application.
-
Discovery of delivery systems that can promote effective ASOs cellular uptake in the immune system.
-
Studies of stability and antigen-ASOs compatibility in vaccine formulations.
-
Immunotoxicity studies to discover potential consequences of immune overstimulation.
-
Studies of efficacy/safety in different genetic contexts.
5. Concluding Remarks
References
- Rappuoli, R.; Mandl, C.W.; Black, S.; De Gregorio, E. Vaccines for the twenty-first society. Nature Rev. Immunol. 2011, 11, 865–872.
- Finco, O.; Rappuoli, R. Designing vaccines for the twenty-first century society. Front. Immunol. 2014, 5, 12.
- Afrough, B.; Dowall, S.; Hewson, R. Emerging viruses and current strategies for vaccine intervention. Clin. Exp. Immunol. 2019, 196, 157–166.
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963.
- Dormitzer, P.R.; Grandi, G.; Rappuoli, R. Structural vaccinology starts to deliver. Nat. Rev. Microbiol. 2012, 10, 807–813.
- Myhr, A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017, 22, 79–88.
- Ghaffarifar, F. Plasmid DNA vaccines: Where are we now? Drugs Today (Barc) 2018, 54, 315–333.
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The new revolution in nucleic acid vaccines. Semin. Immunol. 2013, 25, 152–159.
- Kramps, T.; Elbers, K. Introduction to RNA Vaccines. Methods Mol. Biol. 2017, 1499, 1–11.
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511.
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389.
- Campbell, J.M.; Bacon, T.A.; Wickstrom, E. Oligodeoxynucleoside phosphorothioate stability in subcellular extracts, culture media, sera and cerebrospinal fluid. J. Biochem. Biophys. Methods 1990, 20, 259–267.
- Hyjek, M.; Figiel, M.; Nowotny, M. RNases H: Structure and mechanism. DNA Repair (Amst). 2019, 84, 102672.
- Stein, C.A.; Castanotto, D. FDA-Approved Oligonucleotide Therapies in 2017. Mol. Ther. 2017, 25, 1069–1075.
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of Erysipelas, with a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487–511.
- Taliaferro, W.H.; Jaroslow, B.N. The restoration of hemolysin formation in x-rayed rabbits by nucleic acid derivatives and antagonists of nucleic acid synthesis. J. Infect. Dis. 1960, 107, 341–350.
- Reist, E.J.; Benitez, A.; Goodman, L. The synthesis of some 5′-thiopentofuranosylpyrimidines. J. Org. Chem. 1964, 29, 554–558.
- Codington, J.F.; Doerr, I.L.; Fox, J.J. Nucleosides. XVIII. Synthesis of 2’-fluorothymidine, 2’-fluorodeoxyuridine, and other 2’-halogeno-2¢-deoxy nucleosides. J. Org. Chem. 1964, 29, 558–564.
- Eckstein, F. Nucleoside phosphorothioates. J. Am. Chem. Soc. 1966, 88, 4292–4294.
- Bobst, A.M.; Rottman, F.; Cerutti, P.A. Effect of the methylation of the 2’-hydroxyl groups in polyadenylic acid on its structure in weakly acidic and neutral solutions and on its capability to form ordered complexes with polyuridylic acid. J. Mol. Biol. 1969, 46, 221–234.
- Braun, W.; Nakano, M. Influence of oligodeoxyribonucleotides on early events in antibody formation. Proc. Soc. Exp. Biol. Med. 1965, 119, 701–707.
- Braun, W.; Nakano, M. Antibody formation: Stimulation by polyadenylic and polycytidylic acids. Science 1967, 157, 819–821.
- Steinberg, A.D.; Baron, S.; Talal, N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc. Natl. Acad. Sci. USA 1969, 63, 1102–1107.
- Field, A.K.; Tytell, A.A.; Lampson, G.P.; Hilleman, M.R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl. Acad. Sci. USA 1967, 58, 1004–1010.
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738.
- Tokunaga, T.; Yamamoto, H.; Shimada, S.; Abe, H.; Fukuda, T.; Fujisawa, Y.; Furutani, Y.; Yano, O.; Kataoka, T.; Sudo, T.; et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J. Natl. Cancer Inst. 1984, 72, 955–962.
- Yamamoto, S.; Yamamoto, T.; Shimada, S.; Kuramoto, E.; Yano, O.; Kataoka, T.; Tokunaga, T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol. Immunol. 1992, 36, 983–997.
- Kuramoto, E.; Yano, O.; Kimura, Y.; Baba, M.; Makino, T.; Yamamoto, S.; Yamamoto, T.; Kataoka, T.; Tokunaga, T. Oligonucleotide sequences required for natural killer cell activation. Jpn. J. Cancer Res. 1992, 83, 1128–1131.
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549.
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204.
- Campbell, J.D. Development of the CpG adjuvant 1018: A case study. Methods Mol. Biol. 2017, 1494, 15–27.
- Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002, 20, 709–760.
- Sacher, T.; Knolle, P.; Nichterlein, T.; Arnold, B.; Hämmerling, G.J.; Limmer, A. CpG-ODN-induced inflammation is sufficient to cause T-cell-mediated autoaggression against hepatocytes. Eur. J. Immunol. 2002, 32, 3628–3637.
- Tadema, H.; Abdulahad, W.H.; Lepse, N.; Stegeman, C.A.; Kallenberg, C.G.; Heeringa, P. Bacterial DNA motifs trigger ANCA production in ANCA-associated vasculitis in remission. Rheumatology (Oxford) 2011, 50, 689–696.
- Guerrier, T.; Youinou, P.; Pers, J.O.; Jamin, C. TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J. Autoimmun. 2012, 39, 173–179.
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284.
- Stephenson, M.L.; Zamecnik, P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 285–288.
- Donis-Keller, H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979, 7, 179–192.
- Simons, R.W.; Kleckner, N. Translational control of IS10 transposition. Cell 1983, 34, 683–691.
- Izant, J.G.; Weintraub, H. Inhibition of thymidine kinase gene expression by anti-sense RNA: A molecular approach to genetic analysis. Cell 1984, 36, 1007–1015.
- Harland, R.; Weintraub, H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J. Cell Biol. 1985, 101, 1094–1099.
- Melton, D.A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc. Natl. Acad. Sci. USA 1985, 82, 144–148.
- Matsukura, M.; Zon, G.; Shinozuka, K.; Robert-Guroff, M.; Shimada, T.; Stein, C.A.; Mitsuya, H.; Wong-Staal, F.; Cohen, J.S.; Broder, S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc. Natl. Acad. Sci. USA 1989, 86, 4244–4248.
- Sinha, N.D.; Biernat, J.; McManus, J.; Köster, H. Polymer support oligonucleotide synthesis XVIII: Use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984, 12, 4539–4557.
- Usman, N.; Ogilvie, K.K.; Jiang, M.Y.; Cedergren, R.J. The automated chemical synthesis of long oligoribuncleotides using 2’-O-silylated ribonucleoside 3’-O-phosphoramidites on a controlled-pore glass support: Synthesis of a 43-nucleotide sequence similar to the 3’-half molecule of an Escherichia coli formylmethionine tRNA. J. Am. Chem. Soc. 1987, 109, 7845–7854.
- Walder, J.A.; Walder, R.Y. Nucleic acid hybridization and amplification method for detection of specific sequences in which a complementary labeled nucleic acid probe is cleaved. U.S. Patent 5,403,711, 4 April 1995.
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355.
- Roehr, B. Fomivirsen approved for CMV retinitis. J. Int. Assoc. Physicians AIDS Care 1998, 4, 14–16.
- Wong, E.; Goldberg, T. Mipomersen (kynamro): A novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P. T. 2014, 39, 119–122.
- Aartsma-Rus, A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid Ther. 2017, 27, 67–69.
- Yu, E.Y.; Ellard, S.L.; Hotte, S.J.; Gingerich, J.R.; Joshua, A.M.; Gleave, M.E.; Chi, K.N. A randomized phase 2 study of a HSP27 targeting antisense, apatorsen with prednisone versus prednisone alone, in patients with metastatic castration resistant prostate cancer. Invest. New Drugs 2018, 36, 278–287.
- Spigel, D.R.; Shipley, D.L.; Waterhouse, D.M.; Jones, S.F.; Ward, P.J.; Shih, K.C.; Hemphill, B.; McCleod, M.; Whorf, R.C.; Page, R.D.; et al. A Randomized, Double-Blinded, Phase II Trial of Carboplatin and Pemetrexed with or without Apatorsen (OGX-427) in Patients with Previously Untreated Stage IV Non-Squamous-Non-Small-Cell Lung Cancer: The SPRUCE Trial. Oncologist 2019, 24, e1409–e1416.
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704.
- Nan, Y.; Zhang, Y.J. Antisense Phosphorodiamidate Morpholino Oligomers as Novel Antiviral Compounds. Front Microbiol. 2018, 9, 750.
- Shen, X.; Corey, D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018, 46, 1584–1600.
- Rüger, J.; Ioannou, S.; Castanotto, D.; Stein, C.A. Oligonucleotides to the (Gene) Rescue: FDA Approvals 2017-2019. Trends Pharmacol. Sci. 2020, 41, 27–41.
- Goudsmit, J.; Geelen, J.; Keulen, W.; Notermans, D.; Kuiken, C.; Ramautarsing, C.; Smit, L.; Koole, L.; van Genderen, M.; Buck, H.; et al. Characterization of the African HIV-1 isolate CBL-4 (RUT) by partial sequence analysis and virus neutralization with peptide antibody and antisense phosphate-methylated DNA. AIDS 1990, 4, 559–564.
- Buck, H.M.; Koole, L.H.; van Genderen, M.H.; Smit, L.; Geelen, J.L.; Jurriaans, S.; Goudsmit, J. Phosphate-methylated DNA aimed at HIV-1 RNA loops and integrated DNA inhibits viral infectivity. Science 1990, 248, 208–212.
- Moody, H.M.; Quaedflieg, P.J.L.M.; Koole, L.H.; van Genderen, M.H.P.; Buck, H.M.; Smit, L.; Jurriaans, S.; Geelen, J.L.M.C.; Goudsmit, J. Inhibition of HIV-1 Infectivity by Phosphate-Methylated DNA: Retraction. Science 1990, 250, 125–126.
- Humphreys, R.E.; Hillman, G.G.; von Hofe, E.; Xu, M. Forcing tumor cells to present their own tumor antigens to the immune system: A necessary design for an efficient tumor immunotherapy. Cell. Mol. Immunol. 2004, 1, 180–185.
- Baskar, S.; Azarenko, V.; Garcia Marshall, E.; Hughes, E.; Ostrand-Rosenberg, S. MHC class II-transfected tumor cells induce long-term tumor-specific immunity in autologous mice. Cell Immunol. 1994, 155, 123–133.
- Clements, V.K.; Baskar, S.; Armstrong, T.D.; Ostrand-Rosenberg, S. Invariant chain alters the malignant phenotype of MHC class II+ tumor cells. J. Immunol. 1992, 149, 2391–2396.
- Qiu, G.; Goodchild, J.; Humphreys, R.E.; Xu, M. Cancer immunotherapy by antisense suppression of Ii protein in MHC-class-II-positive tumorcells. Cancer Immunol. Immunother. 1999, 48, 499–506.
- Lu, X.; Kallinteris, N.L.; Li, J.; Wu, S.; Li, Y.; Jiang, Z.; Hillman, G.G.; Gulfo, J.V.; Humphreys, R.E.; Xu, M. Tumor immunotherapy by converting tumor cells to MHC class II-positive, Ii protein-negative phenotype. Cancer Immunol. Immunother. 2003, 52, 592–598.
- Hillman, G.G.; Kallinteris, N.L.; Li, J.; Wang, Y.; Lu, X.; Li, Y.; Wu, S.; Wright, J.L.; Slos, P.; Gulfo, J.V.; et al. Generating MHC Class II+/Ii- phenotype after adenoviral delivery of both an expressible gene for MHC Class II inducer and an antisense Ii-RNA construct in tumor cells. Gene Ther. 2003, 10, 1512–1518.
- Rubenstein, M.; Hollowell, C.M.; Guinan, P. Differentiated prostatic antigen expression in LNCaP cells following treatment with bispecific antisense oligonucleotides directed against BCL-2 and EGFR. Med. Oncol. 2012, 29, 835–841.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. Inhibition of BCL2 by antisense oligonucleotides is followed by a compensatory suppression of caspase-3 in LNCaP cells. Eur. J. Clin. Med. Oncol. 2011, 3, 1–6.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. Additional compensatory mechanisms altering antisense oligonucleotide suppression of BCL2: Effects upon AKT1 and STAT3. In Vivo 2014, 28, 867–870.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. In LNCaP cells enhanced expression of the androgen receptor compensates for BCL2 suppression by antisense oligonucleotides. Ther. Adv. Urology 2011, 3, 73–79.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. In LNCaP cells enhanced expression of both androgen receptor and co-stimulatory protein p300 compensate for antisense oligonucleotide suppression of BCL2. Ther. Adv. Urology 2011, 3, 243–250.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. Increased expression of the androgen receptor with p300 and IL-6 coactivators compensate for oligonucleotide suppression of BCL2. No increased CREBBP or IL-4 expression. Ther. Adv. Urology 2013, 5, 85–93.
- Rubenstein, M.; Hollowell, C.M.P.; Guinan, P. Following inhibition of BCL2 by antisense oligonucleotides compensatory suppression of apoptosis involves the direct signal transduction pathway of LNCaP cell. Online J. Apoptosis 2015, 4, 1–10.
- Rubenstein, M.; Hollowell, C.M.; Guinan, P. Suppression of BCL2 by Antisense Oligonucleotides and Compensation by Non-Targeted Genes May Enhance Tumor Proliferation. In Vivo 2015, 29, 687–693.
- Tsamandas, A.C.; Kardamakis, D.; Ravazoula, P.; Zolota, V.; Salakou, S.; Tepetes, K.; Kalogeropoulou, C.; Tsota, I.; Kourelis, T.; Makatsoris, T.; et al. The potential role of TGFbeta1, TGFbeta2 and TGFbeta3 protein expression in colorectal carcinomas. Correlation with classic histopathologic factors and patient survival. Strahlenther. Onkol. 2004, 180, 201–208.
- Dallas, S.L.; Zhao, S.; Cramer, S.D.; Chen, Z.; Peehl, D.M.; Bonewald, L.F. Preferential production of latent transforming growth factor beta-2 by primary prostatic epithelial cells and its activation by prostate-specific antigen. J. Cell Physiol. 2005, 202, 361–370.
- Polak, M.E.; Borthwick, N.J.; Gabriel, F.G.; Johnson, P.; Higgins, B.; Hurren, J.; McCormick, D.; Jager, M.J.; Cree, I.A. Mechanisms of local immunosuppression in cutaneous melanoma. Br. J. Cancer 2007, 96, 1879–1887.
- Vagenas, K.; Spyropoulos, C.; Gavala, V.; Tsamandas, A.C. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: Correlation with prognostics factors and patient survival. J. Surg. Res. 2007, 139, 182–188.
- Jeon, H.S.; Jen, J. TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 417–419.
- Schlingensiepen, K.H.; Jaschinski, F.; Lang, S.A.; Moser, C.; Geissler, E.K.; Schlitt, H.J.; Kielmanowicz, M.; Schneider, A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011, 102, 1193–1200.
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767.
- Tzai, T.S.; Shiau, A.L.; Liu, L.L.; Wu, C.L. Immunization with TGF-beta antisense oligonucleotide-modified autologous tumor vaccine enhances the antitumor immunity ofMBT-2 tumor-bearing mice through upregulation of MHC clas I and Fas expressions. Anticancer Res. 2000, 20, 1557–1562.
- Schneider, T.; Becker, A.; Ringe, K.; Reinhold, A.; Firsching, R.; Sabel, B.A. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J. Neuroimmunol. 2008, 195, 21–27.
- Nemunaitis, J.; Dillman, R.O.; Schwarzenberger, P.O.; Senzer, N.; Cunningham, C.; Cutler, J.; Tong, A.; Kumar, P.; Pappen, B.; Hamilton, C.; et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 4721–4730.
- Giaccone, G.; Bazhenova, L.A.; Nemunaitis, J.; Tan, M.; Juhász, E.; Ramlau, R.; van den Heuvel, M.M.; Lal, R.; Kloecker, G.H.; Eaton, K.D.; et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer 2015, 51, 2321–2329.
- Schlingensiepen, K.H.; Fischer-Blass, B.; Schmaus, S.; Ludwig, S. Antisense therapeutics for tumor treatment: The TGF-beta2 inhibitor AP 12009 in clinical development against malignant tumors. Recent Results Cancer Res. 2008, 177, 137–150.
- Vallières, L. Trabedersen, a TGFbeta2-specific antisense oligonucleotide for the treatment of malignant gliomas and other tumors overexpressing TGFbeta2. IDrugs 2009, 12, 445–453.
- D’Cruz, O.J.; Qazi, S.; Hwang, L.; Ng, K.; Trieu, V. Impact of targeting transforming growth factor β-2 with antisense OT-101 on the cytokine and chemokine profile in patients with advanced pancreatic cancer. Onco Targets Ther. 2018, 11, 2779–2796.
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: Results of randomized and controlled phase IIb study. Neuro Oncol. 2011, 13, 132–142.
- Papachristodoulou, A.; Silginer, M.; Weller, M.; Schneider, H.; Hasenbach, K.; Janicot, M.; Roth, P. Therapeutic targeting of TGF-β ligands in glioblastoma using novel antisense oligonucleotides reduces the growth of experimental gliomas. Clin. Cancer Res. 2019, 25, 7189–7201.
- Schillaci, R.; Salatino, M.; Cassataro, J.; Proietti, C.J.; Giambartolomei, G.H.; Rivas, M.A.; Carnevale, R.P.; Charreau, E.H.; Elizalde, P.V. Immunization with murine breast cancer cells treated with antisense oligodeoxynucleotides to type I insulin-like growth factor receptor induced an antitumoral effect mediated by a CD8+ response involving Fas/Fas ligand cytotoxic pathway. J. Immunol. 2006, 176, 3426–3437.
- Miguel, A.; Sendra, L.; Noé, V.; Ciudad, C.J.; Dasí, F.; Hervas, D.; Herrero, M.J.; Aliño, S.F. Silencing of Foxp3 enhances the antitumor efficacy of GM-CSF genetically modified tumor cell vaccine against B16 melanoma. Onco Targets Ther. 2017, 10, 503–514.
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. Chem. Med. Chem. 2013, 8, 360–376.
- Batista-Duharte, A.; Téllez-Martínez, D.; Fuentes, D.L.P.; Carlos, I.Z. Molecular adjuvants that modulate regulatory T cell function in vaccination: A critical appraisal. Pharmacol. Res. 2018, 129, 237–250.
- Ripple, M.J.; You, D.; Honnegowda, S.; Giaimo, J.D.; Sewell, A.B.; Becnel, D.M.; Cormier, S.A. Immunomodulation with IL-4Rα antisense oligonucleotide prevents respiratory syncytial virus-mediated pulmonary disease. J. Immunol. 2010, 185, 4804–4811.
- Zhang, J.; Liu, N.; Lu, Y.; Huang, Z.; Zang, Y.; Chen, J.; Zhang, J.; Ding, Z. Phosphorothioated antisense oligodeoxynucleotide suppressing interleukin-10 is a safe and potent vaccine adjuvant. Vaccine 2019, 37, 4081–4088.
- Li, X.; Yang, L.; Zhao, P.; Yao, Y.; Lu, F.; Tu, L.; Liu, J.; Li, Z.; Yu, Y.; Wang, L. Adjuvanticity of a CTLA-4 3’ UTR complementary oligonucleotide for emulsion formulated recombinant subunit and inactivated vaccines. Vaccine 2017, 35, 2379–2389.
- Li, Z.; Song, Y.; Cui, C.; Lan, Y.; Li, X.; Liu, Y.; Lu, F.; Zhang, Y.; Yu, Y.; Wang, L. A LAG3-interfering oligonucleotide acts as an adjuvant to enhance the antibody responses induced by recombinant protein vaccines and inactivated influenza virus vaccines. Appl. Microbiol. Biotechnol. 2019, 103, 6543–6557.
- Akl, M.R.; Ayoub, N.M. Tumor cell transformation using antisense oligonucleotide. Methods Mol. Biol. 2014, 1139, 259–268.
- Gajewski, T.F.; Meng, Y.; Blank, C.; Brown, I.; Kacha, A.; Kline, J.; Harlin, H. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 2006, 213, 131–145.
- Pedersen, L.; Hagedorn, P.H.; Koch, T. Identifying Suitable Target Regions and Analyzing Off-Target Effects of Therapeutic Oligonucleotides. Methods Mol. Biol. 2019, 2036, 261–282.
- Batista-Duharte, A.; Martínez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624.
- Schoch, K.M.; Miller, T.M. Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron 2017, 94, 1056–1070.
- Gagnon, K.T.; Corey, D.R. Guidelines for experiments using antisense oligonucleotides and double-stranded RNAs. Nucleic Acid Ther. 2019, 29, 116–122.




