
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Papucci | + 974 word(s) | 974 | 2020-04-27 04:31:35 | | | |
| 2 | Nicole Yin | + 3 word(s) | 977 | 2020-10-28 10:29:47 | | |
Video Upload Options
Gastric Cancer (GC) is turning out today to be one of the most important welfare issues for both Asian and European countries. Indeed, while the vast majority of the disease burden is located in China and in Pacific and East Asia, GC in European countries still account for about 100.000 deaths per year. With this review article, we aimed to focus the attention on one of the most complex cellular pathways involved in GC proliferation, invasion, migration and metastasis: the MAP Kinases. Such large kinases family is to date constantly studied, since their discovery more than 30 years ago, due to the important role that it plays in the regulation of physiological and pathological processes. Interactions with other cellular proteins as well as miRNAs and lncRNAs may modulate their expression influencing the cellular biological features. Here, we summarize the most important and recent studies involving MAPK in GC. At the same time, we need to underlie that, differently from cancers arising from other tissues, where MAPK pathways seems to be a gold target for anticancer therapies, GC seems to be unique in any aspect. Our aim is to review the current knowledge in MAPK pathways alterations leading to GC, including H. pylori MAPK-triggering to derail from gastric normal epithelium to GC and to encourage researches involved in MAPK signal transduction, that seems to definitely sustain GC development.
1. Introduction
In recent years, ERK-MAPK relationships have been abundantly studied, since mutations and/or alterations of activation pathways has been associated with neoplastic phenotypes of a large number of human tumor cells [1].
2. The ERK/MAPK Pathway in Gastric Cancer
Various studies have demonstrated that the ERK/MAPK pathways are involved in the regulation of cell motility both in GC and in normal epithelia. Indeed, ERK regulates the activity of MMPs in GC, thus influencing cell migration and invasiveness [2]. Moreover, the secreted protein angiopoietin-like-4 (ANGPTL4), which is induced by hypoxia, exerts various effects on the neoplastic progression in scirrhous gastric carcinoma. GC cells may acquire resistance to anoikis through the activation of ANGPTL4-mediated FAK/Src/PI3K-Akt/ERK signaling, inducing the development of peritoneal metastases [3]. There are various studies that have correlated the alteration of the ERK/MEK pathway with the processes of invasion and metastasis; such processes involve motility and cell adhesion and epidermal growth factor receptor (EGFR)-induced disassembly of focal adhesions which is regulated by activating the ERK/MAPK pathways [4]. The p38 pathway turns out to be deregulated in many cancers as well. p38 signaling is involved in the regulation of the epithelial-to-mesenchymal transition (EMT) and in the production of reactive oxygen species (ROS) triggered by EMT which are significantly generated in GCs. One of the targets activated by ROS is EGFR whose overexpression has been linked to lymph node diffusion and consequently to a worse prognosis in GC. These studies have demonstrated the involvement of the EGFR/Ras/MAPK signaling pathway in the activation of NF-κB, in the induction of the cyclooxygenase-2 (COX-2) and in the proliferation of GC cells. NF-κB stimulates the transcription of COX-2, a regulator of cell proliferation. Upregulation of COX-2 facilitates the development of cancer and reduces apoptosis. High levels of COX-2 protein and mRNA have been detected in tissues of patients with GC. It is evident how the EGFR/Ras/MAPK signaling pathway is involved in the activation of NF-κB, through COX-2 induction, and in the stimulation of GC cells proliferation. Han et al. found that lycopene inhibits cell proliferation and induces cell apoptosis by reducing the levels of ROS, and thereby inhibiting ROS-activated EGFR/Ras/ERK and p38 MAPK signaling pathways and suppressing NF-κB p50/p50-mediated COX-2 gene expression in the Caucasian GC cell lines AGS [5]. On the other hand, a large number of studies on GC have shown that the MAPK pathway is associated with apoptosis and autophagy [6]. Liu et al. used ginsenoside Rg5, a rare saponin belonging to the protopanaxadiol ginsenoside family, used as a therapy against many types of cancers, in order to demonstrate its anticancer effect also in GC [7]. In this case Rg5 led to a ROS increase and activated the MAPK pathways, such as those involving p38 and JNK, that works as cancer suppressor, promoting apoptosis autophagy and blocking the cell cycle during the G2/M transition. Also, ERK activation contributed to ROS-associated Rg5-induced GC death. Finally, it was reported the special AT-rich sequence-binding protein 2 (SATB2) as a new tumor-suppressive gene playing an important role in many cancers, including GC. Downregulation of SATB2 in patients with GC was associated with shortened survival and, in the gastric cancer cell line MGC-803, the overexpression of SATB2 was able to repress ERK5 expression, while activation of ERK5 restored the SATB2-induced inhibition of proliferation and migration [8][9]. Even though we reported few examples of the relationships between metastasis formation and the abnormal activation of MAPKs in GC, describing the complex interconnection leading to anoikis resistance, cell adhesion loosening, MMPs secretion and activation, increased proliferation and migration, many efforts need to be still spent in order to elucidate every single pathway and thus uncover new possible targets for future therapies. The main pathways reported above were summarized in Figure 1.
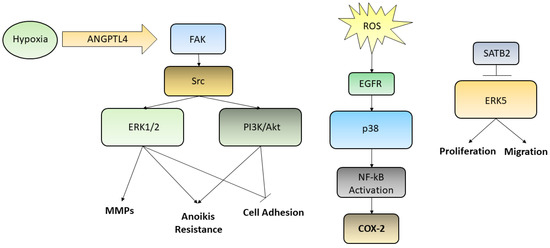
Figure 1. Although several kinases pathways are deregulated in GC, we reported here three ways by which ERK1/2, PI3K/Akt, p38, and ERK5 are activated. Hypoxic condition may trigger, through the ANGPTL4/FAK/Src/ERK1/2–PI3K/Akt axis, events aimed to increase GC cells metastatic potential, such as induction of anoikis resistance, MMPs secretion, loosening of cell adhesion; ROS, influencing EGFR and p38, are able to activate NF-kB translocation into the nucleus leading to the transcription of COX-2 gene; SATB2, which is commonly downregulated in poor prognosis GC patients, being able to inhibit ERK5, is capable to decrease cell proliferation and migration, acting as a tumor suppressor transcription factor.
References
- Amardeep S. Dhillon; S Hagan; Oliver Rath; Walter Kolch; MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279-3290, 10.1038/sj.onc.1210421.
- Hafeza Akter; Min Park; Oh-Seung Kwon; Eun Joo Song; Won-Sang Park; Min-Jung Kang; Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumor Biology 2015, 36, 6053-6062, 10.1007/s13277-015-3282-9.
- Koichi Baba; Yoshihiko Kitajima; Shuusuke Miyake; Jun Nakamura; Kota Wakiyama; Hirofumi Sato; Keiichiro Okuyama; Hiroshi Kitagawa; Tomokazu Tanaka; Masatsugu Hiraki; et al.Kazuyoshi YanagiharaHirokazu Noshiro Hypoxia-induced ANGPTL4 sustains tumour growth and anoikis resistance through different mechanisms in scirrhous gastric cancer cell lines.. Scientific Reports 2017, 7, 11127, 10.1038/s41598-017-11769-x.
- Mei Yang; Changzhi Huang; Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World Journal of Gastroenterology 2015, 21, 11673-11679, 10.3748/wjg.v21.i41.11673.
- Hwana Han; Joo Weon Lim; Hyeyoung Kim; Lycopene Inhibits Activation of Epidermal Growth Factor Receptor and Expression of Cyclooxygenase-2 in Gastric Cancer Cells.. Nutrients 2019, 11, 2113, 10.3390/nu11092113.
- Weimin Li; Mengdi Fan; Yina Chen; Qian Zhao; Caiyun Song; Ye Yan; Yin Jin; Zhiming Huang; Chunjing Lin; Jiansheng Wu; et al. Melatonin Induces Cell Apoptosis in AGS Cells Through the Activation of JNK and P38 MAPK and the Suppression of Nuclear Factor-Kappa B: a Novel Therapeutic Implication for Gastric Cancer. Cellular Physiology and Biochemistry 2015, 37, 2323-2338, 10.1159/000438587.
- Yannan Liu; Daidi Fan; Ginsenoside Rg5 induces G2/M phase arrest, apoptosis and autophagy via regulating ROS-mediated MAPK pathways against human gastric cancer.. Biochemical Pharmacology 2019, 168, 285-304, 10.1016/j.bcp.2019.07.008.
- Liucheng Wu; Jiansi Chen; Yuzhou Qin; Xianwei Mo; Minwei Huang; Haiming Ru; Yang Yang; Jungang Liu; Yuan Lin; SATB2 suppresses gastric cancer cell proliferation and migration. Tumor Biology 2015, 37, 4597-4602, 10.1007/s13277-015-4282-5.
- Barbara Stecca; Elisabetta Rovida; Impact of ERK5 on the Hallmarks of Cancer.. International Journal of Molecular Sciences 2019, 20, 1426, 10.3390/ijms20061426.




