Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kok Yong Chin | + 1837 word(s) | 1837 | 2021-06-02 05:17:10 | | | |
| 2 | Bruce Ren | -21 word(s) | 1816 | 2021-06-02 09:36:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chin, K.Y. Propolis in Protecting the Skeleton. Encyclopedia. Available online: https://encyclopedia.pub/entry/10406 (accessed on 07 February 2026).
Chin KY. Propolis in Protecting the Skeleton. Encyclopedia. Available at: https://encyclopedia.pub/entry/10406. Accessed February 07, 2026.
Chin, Kok Yong. "Propolis in Protecting the Skeleton" Encyclopedia, https://encyclopedia.pub/entry/10406 (accessed February 07, 2026).
Chin, K.Y. (2021, June 02). Propolis in Protecting the Skeleton. In Encyclopedia. https://encyclopedia.pub/entry/10406
Chin, Kok Yong. "Propolis in Protecting the Skeleton." Encyclopedia. Web. 02 June, 2021.
Copy Citation
Chronic inflammation and oxidative stress are two major mechanisms leading to the imbalance between bone resorption and bone formation rate, and subsequently, bone loss. Thus, functional foods and dietary compounds with antioxidant and anti-inflammatory could protect skeletal health. This review aims to examine the current evidence on the skeletal protective effects of propolis, a resin produced by bees, known to possess antioxidant and anti-inflammatory activities.
bee wax
honeybees
periodontitis
osteoblast
osteoclast
osteopenia
osteoporosis
1. Introduction
The skeletal system consisting of dense connective tissues, mainly bone, is metabolically active and functionally diverse. It undergoes modelling (construction) and remodelling (reconstruction) process in response to stimuli throughout our lifetime [1][2]. Bone loss associated with age is often a result of defective bone remodelling. Bone remodelling refers to the skeletal reparative process, whereby small areas of bone are resorbed by osteoclasts and replaced by osteoblasts to prevent the accumulation of microfractures and preserve mineral homeostasis by releasing calcium and phosphorus into the circulation. This tightly regulated process replaces 10% of the bone annually and ensures that skeletal structural integrity, mass and mechanical strength are preserved [3][4].
Many endogenous and exogenous factors influence the bone remodelling process. Among the factors is oxidative stress resulted from an imbalance between oxidants [reactive oxygen species (ROS) and reactive nitrogen species (RNS)] and antioxidants (enzymatic and non-enzymatic) [5][6]. Oxidative stress favours osteoclast formation (osteoclastogenesis) and bone resorption while hampering osteoblast formation (osteoblastogenesis) and bone formation, leading to bone loss [7][8][9]. These skeletal effects are achieved by activating signalling pathways, such as mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERK1/2), c-Jun-N terminal kinase (JNK), and p38 MAPK [10][11][12]. Given the role of oxidative stress in the development of bone loss, antioxidants present in food could have beneficial skeletal effects. Antioxidants improve the survival and functions of osteoblasts and osteocytes, while suppressing osteoclastogenesis and osteoclast activity [7][10][13][14]. Vitamin C, vitamin E, polyphenols, and other antioxidants have been shown to promote osteoblastogenesis, as well as preventing oxidative stress-induced apoptosis of osteoblasts and osteoclastogenesis [15][16][17][18].
Inflammation is closely associated with oxidative stress. Phagocytes involved in the immune response synthesise ROS to destroy the invading pathogens. These ROS could diffuse to the surrounding tissues causing damage. Both lipopolysaccharide from the bacterial cell wall and interleukin (IL)-4 synthesised by the immune cells could activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) which generate ROS. In reciprocal, ROS can activate nuclear factor kappa B (NF-κB) signalling pathway and nucleotide-binding and oligomerisation domain-like receptor family pyrin domain containing 3 inflammasome [19][20]. As implicated in inflammatory bowel disease, rheumatoid arthritis and systemic lupus erythematosus, chronic inflammation is a strong risk factor for bone loss. The pro-inflammatory cytokines released by immune cells potentiate inflammation and promote bone resorption and impair bone formation, resulting in accelerated bone loss and increased fracture risk [21][22][23].
Propolis (or bee glue) is a natural resin mixture produced by honeybees from plant parts, buds, and exudates. Given its waxy consistency and mechanical properties, propolis is used by the bees in the construction and repair of hives to protect against foreign predators and weather elements [24][25]. Propolis contains over 300 potentially active ingredients, including coumarins, phenolic aldehydes, steroids, amino acids and polyphenols [26]. Its functions as an immune enhancer, antibacterial, anti-inflammatory, anti-tumour and antioxidant agent have been investigated [27][28]. The antioxidant properties of propolis are contributors to its other biological effects, including chemoprevention [29] and anti-inflammation [30]. With regard to its anti-inflammatory effects, propolis could inhibit the synthesis of prostaglandin E2 and the inducible cyclooxygenase-2 expression [25][31][32]. In a model of carrageenin-induced paw oedema, propolis prevented inflammation probably by inhibiting nitric oxide (NO) production [30]. Regarding its antioxidant effects, propolis prevented DNA damage caused by hydrogen peroxide in fibroblasts [33]. It also inhibits protein nitration, peroxidation of low-density lipoprotein and endothelial NOX expression, and increases endothelial nitric oxide synthase expression [34]. Besides, propolis could enhance antioxidant capacity in animals [35] and humans [36], thereby lowering lipid peroxidation, which is linked to an increased risk of cardiovascular diseases [37][38].
2. Possible Molecular Mechanisms of Propolis in Preserving Skeletal Health
Many studies have shown that antioxidants help to promote osteoblast differentiation, mineralisation and reduce osteoclast activity by directly or indirectly counteracting the effects of oxidants [13][14][39]. Propolis has been shown to possess antioxidant activity [40], and the majority of studies showing a reduction in oxidative stress markers with propolis treatment [41]. Guney et al. [42] reported a reduction in femoral SOD and glutathione levels in rats treated with propolis. The authors suggested that the administration of an exogenous antioxidant could reduce endogenous antioxidant enzyme expression because the components in propolis could scavenge free radicals [42]. Malondialdehyde (MDA) is frequently used as an indicator of oxidative lipid damage. Wiwekowati et al. [43] reported that propolis reduced MDA levels in rats with OTM. Propolis could effectively eliminate free radicals due to the polyphenol content [44]. Flavonoids, one of the polyphenols in propolis, are potent antioxidants capable of scavenging free radicals, thereby shielding the cell membrane from lipid peroxidation [45]. A positive relationship has been established between the flavonoid content and propolis inhibition of MDA [46]. Caffeic acid phenethyl ester (CAPE) has also been linked to the antioxidant properties of propolis [18]. Propolis extracts containing CAPE were more efficient at inhibiting xanthine oxidase and lipoperoxidase activity than propolis extracts lacking CAPE [47]. The presence of caffeic acid and phenyl caffeate is linked to the high antioxidant potential of propolis [48].
M-CSF and RANKL are two essential cytokines regulating osteoclast differentiation. M-CSF ensures the survival and proliferation of osteoclast precursor cells. It also increases the RANK expression in osteoclast precursor cells, ensuring a more efficient response to the RANKL-RANK signalling pathways [49][50][51][52]. Wimolsantirungsri et al. [53] reported decreased RANKL and M-CSF-induced RANK expression in osteoclast precursor cells following propolis treatment, suggesting the inhibition of RANKL-RANK signalling pathway, which would eventually lead to reduced osteoclast differentiation. RANKL activates several transcription factors, including NFAT2, a master regulator of osteoclast differentiation that controls the expression of several osteoclast-specific genes, such as TRAP, cathepsin K, and CTR [54][55][56][57]. Two studies demonstrated that propolis inhibited osteoclast formation from human peripheral blood mononuclear cells [53] and RAW 264.7 cells [58] by inhibiting the expression of NFAT2, cathepsin K and CTR.
Inflammatory cytokines cause bone loss by promoting osteoclast differentiation and maturation directly. These cytokines work together to attract, differentiate, and activate osteoclasts through the NF-κB signalling pathway [59][60][61]. Propolis-incorporated bone implants were shown to downregulate the expression of IL-1β, and TNF-α at the surrounding tissue [62], thereby preventing osteoclast formation and bone resorption that would loosen the implants. Another study on ligature-induced periodontitis failed to reduce circulating inflammatory cytokines by propolis, probably because the rats also presented diabetes and a higher degree of inflammation [63]. Propolis suppressed LPS-induced expression of IL-1β, IL-6, and IL-8 in human periodontal ligament cells [64] and IL-6 in RAW264.7 macrophages [65].
Osteoblasts derived from mesenchymal stem cells in the bone marrow are responsible for the synthesis, secretion, and mineralisation of bone matrix. They also secrete OPG, a RANKL decoy receptor that prevents the binding of RANKL to RANK, thereby halting RANKL signalling and osteoclastogenesis [66]. Propolis was shown to stimulate the proliferation, differentiation and maturation of osteoblasts [67]. Among the many osteoblast markers, OPG expression was also upregulated by propolis, indicating that it could affect RANKL/OPG dynamic or crosstalk between osteoblasts and osteoclasts, thereby suppressing osteoclastogenesis.
BMPs are growth factors belonging to the transforming growth factor-superfamily. Their diverse functions range from regulating bone induction, preservation, and reconstruction to determining non-osteogenic embryological developmental pathways and maintaining adult tissue homeostasis [68]. BMP-2 and 7 are involved in bone development and regeneration during osteoblast differentiation [69]. The role of BMP signalling in polyphenol-mediated bone anabolism has been extensively studied, and several studies have shown that increasing BMP-2 promoter activity and BMP-2 expression increases new bone formation [70][71]. Propolis loaded implants increased the expression of BMP-2 and 7 at the surrounding tissue. This event occurs in parallel with a reduction in inflammatory cytokine expression, increased new bone development around the implant, and enhanced adhesion with the mandibular and implant [62]. The possible molecular mechanisms of propolis are summarised in Figure 1.
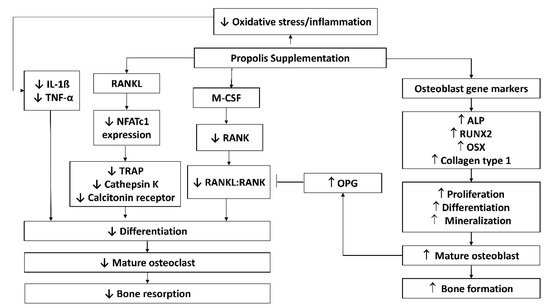
Figure 1. Possible molecular mechanisms of propolis in protecting against bone loss. Abbreviations: ALP, alkaline phosphatase; IL-1β, interleukin-1 beta; M-CSF, macrophage colony-stimulating factor; NFATc1, nuclear factor of activated T-cells; OPG, osteoprotegerin; OSX, osterix; RANK, receptor activator of nuclear factor-kappa B; RANKL, RANK ligand; TNF-α, tumour necrosis factor-alpha; TRAP, tartrate-resistant acid phosphatase.
Based on current evidence and the possible molecular mechanism, propolis has the potential to protect the skeletal system and improve bone remodelling by reducing the expression of inflammatory cytokines responsible for osteoclast differentiation and osteoblast apoptosis, inhibiting RANKL and M-CSF signalling pathway responsible for osteoclast differentiation and maturation and increasing osteoblast proliferation, differentiation, and mineralisation through its increased expression of osteoblast markers. These properties could be useful in the treatment of several medical conditions which promote bone loss or fractures. So far, propolis is already being considered in periodontal healthcare as there are existing clinical trials on the effect of propolis on periodontal health. However, there are no results available.
3. Bioavailability and Safety Concerns of Propolis
Propolis is made up of lipids, waxes, and resins in a complex matrix with a high molecular weight, contributing to its low absorption and bioavailability [72]. The type of polyphenols present and their interactions dictate the synergistic effects and influence the bioavailability of propolis [72]. Digestive instability, poor transcellular efflux in intestinal cells, as well as rapid metabolism and excretion are all thought to play a role in polyphenol bioavailability [45]. Dietary polyphenols cannot be absorbed because they exist as esters, polymers, or glycosylated forms, and must be hydrolysed by intestinal enzymes or colonic microflora before absorption [72]. Poorly absorbed polyphenolic compounds are converted to smaller phenolic acids with improved bioavailability in the intestinal system, owing to the colonic microbiota enzyme activity [73]. Inter-individuality in absorption and metabolism is important as individual microbiota differ. Despite the low absorption percentages of bio-accessible phenolic compounds in propolis, Yesiltas et al. [74] reported that the recovered amounts detected in plasma were still high compared to other food materials like fruits and vegetables.
Propolis and its constituents are generally well-tolerated and non-toxic unless given in huge doses according to clinical studies in mice and humans [44][75][76]. According to Dobrowolski et al. [77], the median lethal dose (LD50) for various propolis sources ranged from 2 to 7.3 g/kg in mice, implying a healthy dosage of 1.4 to 70 mg/day for humans based on a safety factor of 1000. The LD50 of propolis extract given to conscious mice was more than 7.34 g/kg, indicating that the product is generally safe [78][79]. However, it should be noted that propolis toxicity and adverse events were rarely monitored in human trials. Hypersensitivity is a more common side effect of propolis, especially when applied topically, resulting in allergic reactions, swelling, dermatitis, and urticaria [80]. Hsu et al. [80] reported a patient presenting severe swelling of the throat and anaphylactic shock upon topical application of propolis. Severe side effects like laryngeal oedema and anaphylactic shock have been reported infrequently [81] and are rarely attributed to propolis. Propolis sensitivities have been reported in 1.2–6.6% of people with dermatitis [82]. Therefore, consumers should seek medical advice before taking propolis supplements or applying propolis products, despite its positive safety profile.
References
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–S139.
- Seeman, E.; Delmas, P.D. Bone quality—The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261.
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137.
- Baron, R.; Hesse, E. Update on Bone Anabolics in Osteoporosis Treatment: Rationale, Current Status, and Perspectives. J. Clin. Endocrinol. Metab. 2012, 97, 311–325.
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 2005, 146, 728–735.
- Manolagas, S.C. From Estrogen-Centric to Aging and Oxidative Stress: A Revised Perspective of the Pathogenesis of Osteoporosis. Endocr. Rev. 2010, 31, 266–300.
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555.
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235.
- Mann, V.; Huber, C.; Kogianni, G.; Collins, F.; Noble, B. The antioxidant effect of estrogen and Selective Estrogen Receptor Modulators in the inhibition of osteocyte apoptosis in vitro. Bone 2007, 40, 674–684.
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: Involvement of JNK and ERK1/2 signalling. Calcif. Tissue Int. 2015, 96, 335–346.
- Marathe, N.; Rangaswami, H.; Zhuang, S.; Boss, G.R.; Pilz, R.B. Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J. Biol. Chem. 2012, 287, 978–988.
- Plotkin, L.I.; Aguirre, J.I.; Kousteni, S.; Manolagas, S.C.; Bellido, T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J. Biol. Chem. 2005, 280, 7317–7325.
- Jun, J.H.; Lee, S.-H.; Kwak, H.B.; Lee, Z.H.; Seo, S.-B.; Woo, K.M.; Ryoo, H.-M.; Kim, G.-S.; Baek, J.-H. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J. Cell. Biochem. 2008, 103, 1246–1255.
- Romagnoli, C.; Marcucci, G.; Favilli, F.; Zonefrati, R.; Mavilia, C.; Galli, G.; Tanini, A.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like SaOS-2 cells. FEBS J. 2013, 280, 867–879.
- Chin, K.Y.; Ima-Nirwana, S. Vitamin C and Bone Health: Evidence from Cell, Animal and Human Studies. Curr. Drug Targets 2018, 19, 439–450.
- Chin, K.Y.; Ima-Nirwana, S. Olives and bone: A green osteoporosis prevention option. Int. J. Environ. Res. Public Health 2016, 13, 755.
- Wong, S.; Mohamad, N.-V.; Ibrahim, N.; Chin, K.-Y.; Shuid, A.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453.
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Devel. Ther. 2021, 15, 259–275.
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931.
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230.
- Mundy, G.R. Osteoporosis and inflammation. Nutr. Rev. 2007, 65, S147–S151.
- Romas, E.; Gillespie, M.T. Inflammation-induced bone loss: Can it be prevented? Rheum. Dis. Clin. N. Am. 2006, 32, 759–773.
- Smolen, J.S.; Han, C.; van der Heijde, D.M.F.M.; Emery, P.; Bathon, J.M.; Keystone, E.; Maini, R.N.; Kalden, J.R.; Aletaha, D.; Baker, D.; et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: The impacts of remission and tumour necrosis factor blockade. Ann. Rheum. Dis. 2009, 68, 823–827.
- Bankova, V.S.; Castro, S.L.D.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15.
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363.
- de Castro, P.A.; Savoldi, M.; Bonatto, D.; Barros, M.H.; Goldman, M.H.S.; Berretta, A.A.; Goldman, G.H. Molecular scharacterisation of propolis-induced cell death in Saccharomyces cerevisiae. Eukaryot. Cell 2011, 10, 398–411.
- Khalil, M.L. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31.
- Pahlavani, N.; Sedaghat, A.; Bagheri Moghaddam, A.; Mazloumi Kiapey, S.S.; Gholizadeh Navashenaq, J.; Jarahi, L.; Reazvani, R.; Norouzy, A.; Nematy, M.; Safarian, M.; et al. Effects of propolis and melatonin on oxidative stress, inflammation, and clinical status in patients with primary sepsis: Study protocol and review on previous studies. Clin. Nutr. ESPEN 2019, 33, 125–131.
- Kolankaya, D.; Selmanoǧlu, G.; Sorkun, K.; Salih, B. Protective effects of Turkish propolis on alcohol-induced serum lipid changes and liver injury in male rats. Food Chem. 2002, 78, 213–217.
- Tan-no, K.; Nakajima, T.; Shoji, T.; Nakagawasai, O.; Niijima, F.; Ishikawa, M.; Endo, Y.; Sato, T.; Satoh, S.; Tadano, T. Anti-inflammatory Effect of Propolis through Inhibition of Nitric Oxide Production on Carrageenin-Induced Mouse Paw Edema. Biol. Pharm. Bull. 2006, 29, 96–99.
- Krol, W.; Scheller, S.; Czuba, Z.; Matsuno, T.; Zydowicz, G.; Shani, J.; Mos, M. Inhibition of neutrophils’ chemiluminescence by ethanol extract of propolis (EEP) and its phenolic components. J. Ethnopharmacol. 1996, 55, 19–25.
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99.
- Aliyazicioglu, Y.; Demir, S.; Turan, I.; Cakiroglu, T.N.; Akalin, I.; Deger, O.; Bedir, A. Preventive and Protective Effects of Turkish Propolis on H2O2-induced DNA Damage in Foreskin Fibroblast Cell Lines. Acta Biol. Hung. 2011, 62, 388–396.
- Silva, V.; Genta, G.; Möller, M.N.; Masner, M.; Thomson, L.; Romero, N.; Radi, R.; Fernandes, D.C.; Laurindo, F.R.M.; Heinzen, H.; et al. Antioxidant Activity of Uruguayan Propolis. In Vitro and Cellular Assays. J. Agric. Food Chem. 2011, 59, 6430–6437.
- Zhao, J.-Q.; Wen, Y.-F.; Bhadauria, M.; Nirala, S.K.; Sharma, A.; Shrivastava, S.; Shukla, S.; Agrawal, O.P.; Mathur, R. Protective effects of propolis on inorganic mercury induced oxidative stress in mice. Indian J. Exp. Biol. 2009, 47, 264–269.
- Jasprica, I.; Mornar, A.; Debeljak, Ž.; Smolčić-Bubalo, A.; Medić-Šarić, M.; Mayer, L.; Romić, Ž.; Bućan, K.; Balog, T.; Sobočanec, S.; et al. In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J. Ethnopharmacol. 2007, 110, 548–554.
- Kart, A.; Cigremis, Y.; Ozen, H.; Dogan, O. Caffeic acid phenethyl ester prevents ovary ischemia/reperfusion injury in rabbits. Food Chem. Toxicol. 2009, 47, 1980–1984.
- Tekin, I.O.; Sipahi, E.Y.; Comert, M.; Acikgoz, S.; Yurdakan, G. Low-Density Lipoproteins Oxidized After Intestinal Ischemia/Reperfusion in Rats. J. Surg. Res. 2009, 157, e47–e54.
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258.
- Kwon, Y.-S.; Park, D.-H.; Shin, E.-J.; Kwon, M.S.; Ko, K.H.; Kim, W.-K.; Jhoo, J.H.; Jhoo, W.-K.; Wie, M.-B.; Jung, B.D.; et al. Antioxidant propolis attenuates kainate-induced neurotoxicity via adenosine A1 receptor modulation in the rat. Neurosci. Lett. 2004, 355, 231–235.
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223.
- Guney, A.; Karaman, I.; Oner, M.; Yerer, M.B. Effects of Propolis on Fracture Healing: An Experimental Study. Phytother. Res. 2011, 25, 1648–1652.
- Wiwekowati, W.; Ma’ruf, M.T.; Walianto, S.; Sabir, A.; Widyadharma, I.P.E. Indonesian Propolis Reduces Malondialdehyde Level and Increase Osteoblast Cell Number in Wistar Rats with Orthodontic Tooth Movement. Open Access Maced. J. Med. Sci. 2020, 8, 100–104.
- Cao, G.; Ying, P.; Yan, B.; Xue, W.; Li, K.; Shi, A.; Sun, T.; Yan, J.; Hu, X. Pharmacokinetics, safety, and tolerability of single and multiple-doses of pinocembrin injection administered intravenously in healthy subjects. J. Ethnopharmacol. 2015, 168, 31–36.
- Weaver, C.M.; Barnes, S.; Wyss, J.M.; Kim, H.; Morré, D.M.; Morré, D.J.; Simon, J.E.; Lila, M.A.; Janle, E.M.; Ferruzzi, M.G. Botanicals for age-related diseases: From field to practice. Am. J. Clin. Nutr. 2008, 87, 493S–497S.
- Moreno, M.I.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114.
- Russo, A.; Longo, R.; Vanella, A. Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002, 73 (Suppl. 1), S21–S29.
- Hamasaka, T.; Kumazawa, S.; Fujimoto, T.; Nakayama, T. Antioxidant Activity and Constituents of Propolis Collected in Various Areas of Japan. Food Sci. Technol. Res. 2004, 10, 86–92.
- Dougall, W.C.; Glaccum, M.; Charrier, K.; Rohrbach, K.; Brasel, K.; De Smedt, T.; Daro, E.; Smith, J.; Tometsko, M.E.; Maliszewski, C.R.; et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999, 13, 2412–2424.
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323.
- Wiktor-Jedrzejczak, W.; Bartocci, A.; Ferrante, A.W., Jr.; Ahmed-Ansari, A.; Sell, K.W.; Pollard, J.W.; Stanley, E.R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 1990, 87, 4828–4832.
- Yoshida, H.; Hayashi, S.; Kunisada, T.; Ogawa, M.; Nishikawa, S.; Okamura, H.; Sudo, T.; Shultz, L.D.; Nishikawa, S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 1990, 345, 442–444.
- Wimolsantirungsri, N.; Makeudom, A.; Louwakul, P.; Sastraruji, T.; Chailertvanitkul, P.; Supanchart, C.; Krisanaprakornkit, S. Inhibitory effect of Thai propolis on human osteoclastogenesis. Dent. Traumatol. 2018, 34, 237–244.
- Kim, Y.; Sato, K.; Asagiri, M.; Morita, I.; Soma, K.; Takayanagi, H. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J. Biol. Chem. 2005, 280, 32905–32913.
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.-M.; Lee, S.-H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216.
- Matsumoto, M.; Kogawa, M.; Wada, S.; Takayanagi, H.; Tsujimoto, M.; Katayama, S.; Hisatake, K.; Nogi, Y. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J. Biol. Chem. 2004, 279, 45969–45979.
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.-I.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901.
- Pileggi, R.; Antony, K.; Johnson, K.; Zuo, J.; Shannon Holliday, L. Propolis inhibits osteoclast maturation. Dent. Traumatol. 2009, 25, 584–588.
- Jimi, E.; Takakura, N.; Hiura, F.; Nakamura, I.; Hirata-Tsuchiya, S. The Role of NF-κB in Physiological Bone Development and Inflammatory Bone Diseases: Is NF-κB Inhibition “Killing Two Birds with One Stone”? Cells 2019, 8, 1636.
- Yan, S.-D.; Huang, C.-C. The role of tumor necrosis factor-alpha in bone resorption of cholesteatoma. Am. J. Otolaryngol. 1991, 12, 83–89.
- Yellon, R.F.; Leonard, G.; Marucha, P.T.; Craven, R.; Carpenter, R.J.; Lehmann, W.B.; Burleson, J.A.; Kreutzer, D.L. Characterization of cytokines present in middle ear effusions. Laryngoscope 1991, 101, 165–169.
- Somsanith, N.; Kim, Y.-K.; Jang, Y.-S.; Lee, Y.-H.; Yi, H.-K.; Jang, J.-H.; Kim, K.-A.; Bae, T.-S.; Lee, M.-H. Enhancing of Osseointegration with Propolis-Loaded TiO2 Nanotubes in Rat Mandible for Dental Implants. Materials 2018, 11, 61.
- Aral, C.A.; Kesim, S.; Greenwell, H.; Kara, M.; Çetin, A.; Yakan, B. Alveolar bone protective and hypoglycemic effects of systemic propolis treatment in experimental periodontitis and diabetes mellitus. J. Med. Food 2015, 18, 195–201.
- Yuan, X.; Wang, Y.; Shi, B.; Zhao, Y. Effect of propolis on preserving human periodontal ligament cells and regulating pro-inflammatory cytokines. Dent. Traumatol. 2018, 34, 245–253.
- Neiva, K.G.; Catalfamo, D.L.; Holliday, S.; Wallet, S.M.; Pileggi, R. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts. Dent. Traumatol. 2014, 30, 362–367.
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148.
- Lim, Y.K.; Yoo, S.Y.; Jang, Y.Y.; Lee, B.C.; Lee, D.S.; Kook, J.-K. Anti-inflammatory and in vitro bone formation effects of Garcinia mangostana L. and propolis extracts. Food Sci. Biotechnol. 2020, 29, 539–548.
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241.
- Kawai, M.; Bessho, K.; Maruyama, H.; Miyazaki, J.-I.; Yamamoto, T. Simultaneous gene transfer of bone morphogenetic protein (BMP)-2 and BMP-7 by in vivo electroporation induces rapid bone formation and BMP-4 expression. BMC Musculoskelet. Disord. 2006, 7, 62.
- Lin, Y.; Murray, M.A.; Garrett, I.R.; Gutierrez, G.E.; Nyman, J.S.; Mundy, G.; Fast, D.; Gellenbeck, K.W.; Chandra, A.; Ramakrishnan, S. A targeted approach for evaluating preclinical activity of botanical extracts for support of bone health. J. Nutr. Sci. 2014, 3, e13.
- Zhang, J.; Lazarenko, O.P.; Wu, X.; Tong, Y.; Blackburn, M.L.; Gomez-Acevedo, H.; Shankar, K.; Badger, T.M.; Ronis, M.J.J.; Chen, J.-R. Differential effects of short term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PLOS ONE 2012, 7, e35736.
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208.
- Alkhaldy, A.; Edwards, C.A.; Combet, E. The urinary phenolic acid profile varies between younger and older adults after a polyphenol-rich meal despite limited differences in in vitro colonic catabolism. Eur. J. Nutr. 2019, 58, 1095–1111.
- Yesiltas, B.; Capanoglu, E.; Firatligil-Durmus, E.; Sunay, A.E.; Samanci, T.; Boyacioglu, D. Investigating the in-vitro bioaccessibility of propolis and pollen using a simulated gastrointestinal digestion System. J. Apic. Res. 2014, 53, 101–108.
- Bazmandegan, G.; Boroushaki, M.T.; Shamsizadeh, A.; Ayoobi, F.; Hakimizadeh, E.; Allahtavakoli, M. Brown propolis attenuates cerebral ischemia-induced oxidative damage via affecting antioxidant enzyme system in mice. Biomed. Pharm. 2017, 85, 503–510.
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharm. 2017, 8, 412.
- Dobrowolski, J.W.; Vohora, S.B.; Sharma, K.; Shah, S.A.; Naqvi, S.A.; Dandiya, P.C. Antibacterial, antifungal, antiamoebic, antiinflammatory and antipyretic studies on propolis bee products. J. Ethnopharmacol. 1991, 35, 77–82.
- Arvouet-Grand, A.; Lejeune, B.; Bastide, P.; Pourrat, A.; Privat, A.M.; Legret, P. Propolis extract. I. Acute toxicity and determination of acute primary cutaneous irritation index. J. Pharm. Belg. 1993, 48, 165–170.
- Hamza, R.; Elaziz, A.; Diab, A.; El-Aziz, E.-S.A.A. Hyperglycemic effect of Chlorpyrifos, Profenofos and possible ameliorative role of Propolis and ginseng. Scientia Adv. Agric. Biol. 2014, 1, 9–14.
- Hsu, C.-Y.; Chiang, W.-C.; Weng, T.-I.; Chen, W.-J.; Yuan, A. Laryngeal edema and anaphalactic shock after topical propolis use for acute pharyngitis. Am. J. Emerg. Med. 2004, 22, 432–433.
- Li, Y.-J.; Lin, J.-L.; Yang, C.-W.; Yu, C.-C. Acute renal failure induced by a Brazilian variety of propolis. Am. J. Kidney Dis. 2005, 46, e125–e129.
- Walgrave, S.E.; Warshaw, E.M.; Glesne, L.A. Allergic contact dermatitis from propolis. Dermatitis 2005, 16, 209–215.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
02 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




