Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diego Ramón Pérez | + 1193 word(s) | 1193 | 2021-05-25 10:48:13 | | | |
| 2 | Vicky Zhou | Meta information modification | 1193 | 2021-06-01 09:42:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pérez, D.R. Microglia in Neurogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/10342 (accessed on 07 February 2026).
Pérez DR. Microglia in Neurogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/10342. Accessed February 07, 2026.
Pérez, Diego Ramón. "Microglia in Neurogenesis" Encyclopedia, https://encyclopedia.pub/entry/10342 (accessed February 07, 2026).
Pérez, D.R. (2021, June 01). Microglia in Neurogenesis. In Encyclopedia. https://encyclopedia.pub/entry/10342
Pérez, Diego Ramón. "Microglia in Neurogenesis." Encyclopedia. Web. 01 June, 2021.
Copy Citation
Certain pools of microglia are determinant cells in different phases of the generation of new neurons. This sheds light on how cells cooperate in order to fine tune brain organization.
microglia
inflammation
adult neurogenesis
age
neurodegenerative diseases
1. Introduction
Neurogenesis is the formation of new neurons from neural stem cells and is a major event in the development of the neural system. Although neurogenesis is a process that fundamentally takes place in the pre- and postnatal periods, the synthesis of new neurons in mammals is maintained during adult life in certain brain niches [1]. Adult neurogenesis is crucial to consolidate memory and learning and may be altered as a result of aging, neurodegenerative diseases, and other pathological conditions, such as status epilepticus (SE) [2]. Interestingly, neurogenesis is strongly influenced by the action of brain-resident immune cells. In particular, microglia have been revealed as a central actor in the production, maturation, and integration of new neurons into existing circuitry.
2. Role of Microglia in Neurogenesis
Adult neurogenesis and developmental neurogenesis are characterized by different features. Activated microglia regulate normal embryonic neurogenesis by phagocytosis and by secreting molecules such as nitric oxide (NO) and inflammatory cytokines. However, these molecules may be deleterious for adult neurogenesis. In vitro, microglia can produce IL-6, which leads to apoptosis of neuroblasts [3]. However, they can also produce IL-10, which has the opposite effect [4]. Most experiments indicate that microglial cells are neuroprotective. Nevertheless, a relatively small disturbance in CNS homeostasis can contribute to the progression of neurodegeneration, and microglia play an important role in this. For example, in most neurodegenerative diseases, microglia are stimulated with immune factors and Toll-like receptors (TLRs), and this has an impact on the capacity of microglia to phagocytize neurons and protein complexes [5].
Increasing evidence suggests that microglia are determinant cells of neurogenic niches, such as the SVZ and SGZ of the DG in the hippocampus. They have the capacity to guide the differentiation of precursor cells to neurons. An in vitro study showed that neural progenitor cells cultured in a microglia-conditioned media gave rise to a higher proportion of neurons because of soluble factors released by microglia [6].
There is heterogeneity in microglia in neurogenic areas. However, in murine models, different researchers have reported that microglia in these zones are significantly less involved than microglia from adjacent areas [7]. Significantly increased CD68 expression in microglia was also reported in the olfactory bulb of adult wild-type rats [8]. Furthermore, a microglial population expressing Clec7a was shown to be restricted to the DG by immunohistochemistry (IHC) [9].
Several relevant experiments involve microglia cells and neurogenesis. Of these, the work of Kreisel et al. in 2019 is of note [9]. Using IHC, quantitative real-time PCR, and RNA sequencing methods on neural cells extracted from mice brains, they concluded that “microglia cells play an essential role in survival of newly formed neuroblasts, for basal hippocampal neurogenesis and for vascular endothelial growth factor (VEGF)–induced neurogenic enhancement”.
These authors showed that microglia in neurogenic niches have characteristics and properties that differ from those of microglial cells located in other parts of the nervous system, and these allow them to fulfill functions related to neurogenesis. For example, they used a transgenic system for conditional VEGF induction through which they achieved an improvement in hippocampal neurogenesis. They concluded that DG microglia were the only susceptible microglia subpopulation involved in widespread local proliferation and activation.
2.1. Stages of Adult Neurogenesis and the Role of Microglia
There are two paths that adult neural stem cells (ANSCs) may take: they can produce new astroglia under inflammatory conditions or become TAPCs (transient amplifying progenitor cells), neuroblasts, and new neurons, through proliferation, surveillance, differentiation, migration, and maturation [10] (Figure 1). Relevant molecules and regulatory factors, including Pax6, Tbr2, Sox2, and Prox1, participate in all these processes [11][12][13][14]. ANSCs can differentiate into neurons, astrocytes, and oligodendrocytes in cell cultures [15]. In this article, we refer exclusively to neural production.
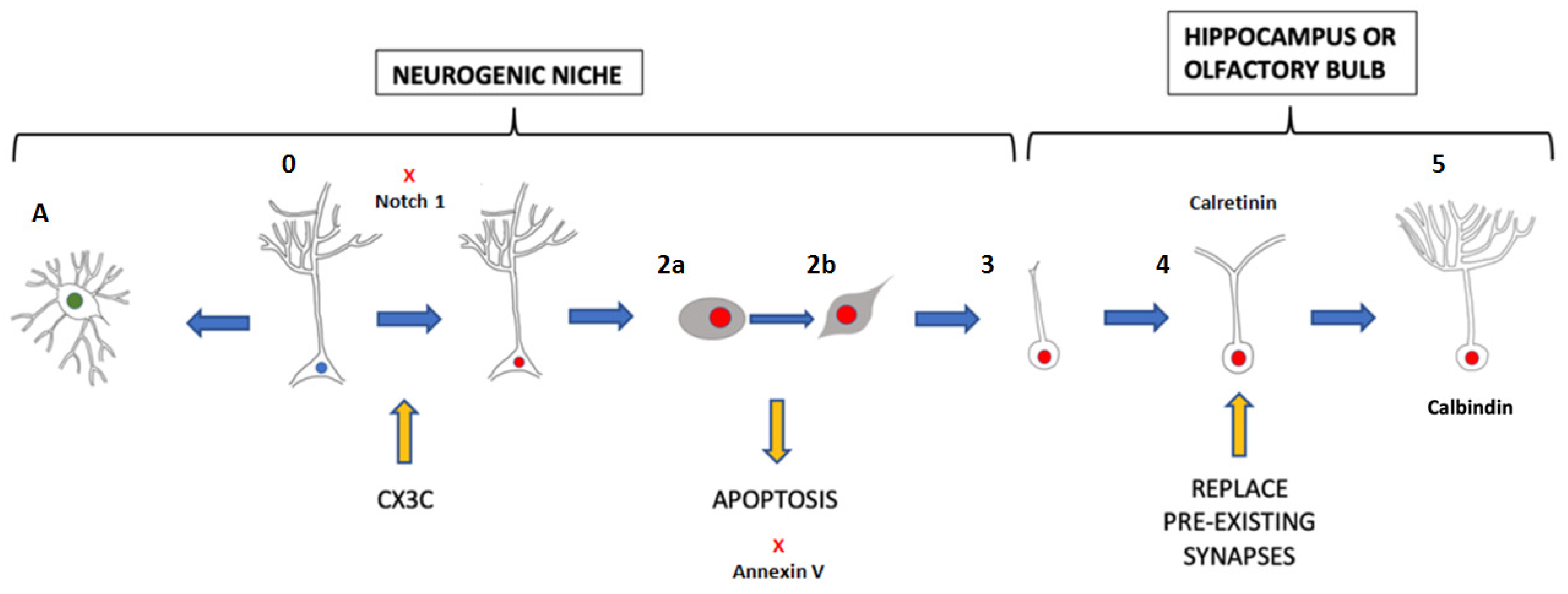 Figure 1. Process of neurogenesis. 0: ANSC in a quiescent state. 1: Type 1 cell. ANSC-activated. 2: Type 2 cell (differentiating 2a and 2b because of their phenotype). TAPC. 3: Type 3 cell. Migratory neuroblast. 4: Immature neuron. 5: Mature neuron. A. Astrocyte. The yellow arrows represent the participation of microglia in this process: microglia are able to activate latent ANSCs by the CX3C chemokine receptor (CX3CR1) in the hippocampus of mice engaged in exercise. They also participate in neurogenesis, inducing apoptosis in some type 2 cells, before the microglia phagocytize them. In addition, newborn cells have to compete with mature neurons and microglia collaborate at this point because they phagocytize weak or less active synapses. Notch1 stimulates the quiescent state, preventing the excessive activation of ANSCs. Annexin V is able to reduce apoptosis and may increase the number of apoptotic TAPCs. Migratory neuroblasts differentiate into immature neurons, which are characterized by a transient expression of calretinin.
Figure 1. Process of neurogenesis. 0: ANSC in a quiescent state. 1: Type 1 cell. ANSC-activated. 2: Type 2 cell (differentiating 2a and 2b because of their phenotype). TAPC. 3: Type 3 cell. Migratory neuroblast. 4: Immature neuron. 5: Mature neuron. A. Astrocyte. The yellow arrows represent the participation of microglia in this process: microglia are able to activate latent ANSCs by the CX3C chemokine receptor (CX3CR1) in the hippocampus of mice engaged in exercise. They also participate in neurogenesis, inducing apoptosis in some type 2 cells, before the microglia phagocytize them. In addition, newborn cells have to compete with mature neurons and microglia collaborate at this point because they phagocytize weak or less active synapses. Notch1 stimulates the quiescent state, preventing the excessive activation of ANSCs. Annexin V is able to reduce apoptosis and may increase the number of apoptotic TAPCs. Migratory neuroblasts differentiate into immature neurons, which are characterized by a transient expression of calretinin.2.2. Recent Discoveries
Díaz-Aparicio et al. (2020) [16] found that the microglial phagocytosis of neural progenitors is essential for neurogenesis. They realized that chronic impairment of microglial phagocytosis decreases adult hippocampal neurogenesis, whereas an acute microglial phagocytosis impairment transiently increases it.
This group reported that phagocytic microglia actively maintains neurogenesis by sensing apoptosis. Interestingly, they proposed that microglial phagocytosis provides a negative feedback loop that is necessary for the long-term maintenance of adult hippocampal neurogenesis.
In addition, they found that the secretome (containing metabolites, miRNAs vesicles, extracellular vesicles, etc.) of phagocytic microglia reduced the most mature neuroblast subpopulation at 28 days in vivo. Therefore, it seems that microglia could regulate neurogenesis through the phagocytosis secretome. However, the secretome of inflammatory microglia is not enough to stimulate microglia to phagocytize neural stem cells [16].
There are some similarities between inflammatory and phagocytic secretomes, such as the content of IL-1, IL-6, and TNF cytokines. Nevertheless, experiments with the secretome of inflammatory microglia did not trigger a reduction in the survival of NPCs [16].
Microglia play a key role in modulating adult neurogenesis, which is not limited to the clearance and removal of cells. They are also involved in neuroprotection and neurodegeneration, and their presence in neurogenic niches, displaying specific characteristics and properties, has been proven. They guide the differentiation of precursor cells into neurons throughout the whole process; they guide the microglial phagocytosis of neural progenitors, which is as essential for neurogenesis; and they remodel synaptic connections in the adult hippocampus, helping to establish functional circuits, which promotes consolidation of long-term memories. Nevertheless, further studies are needed to fully determine microglia involvement in neurogenesis.
3. Conclusions
Neurogenesis can occur in adult humans in certain areas, such as the SVZ and SGZ. Microglia cells play a pivotal role in this process. These cells can promote normal neurogenesis or stop it. This is important in the SGZ for learning and the incorporation of memories and in situations where aberrant neurogenesis is produced, such as during SE. Neurogenesis is also altered in a number of brain pathologies, particularly in neurodegenerative diseases, mainly due to the generation of inflammatory environments. This involves the activation of microglia cells, which phagocytize ANSCs in neurogenic niches.
In summary, further understanding of these processes will allow neuroscientists to better manage the physiopathology of neurodegenerative diseases and, ultimately, to find new targets to prevent neurodegeneration. This will in turn help to reduce the pain that these pathologies cause to patients and their relatives.
References
- Mori, T.; Buffo, A.; Götz, M. The Novel Roles of Glial Cells Revisited: The Contribution of Radial Glia and Astrocytes to Neurogenesis. Curr. Top. Dev. Biol. 2005, 69, 67–99.
- Luo, C.; Koyama, R.; Ikegaya, Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia 2016, 64, 1508–1517.
- Campbell, I.L.; Erta, M.; Lim, S.L.; Frausto, R.; May, U.; Rose-John, S.; Scheller, J.; Hidalgo, J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J. Neurosci. 2014, 34, 2503–2513.
- Matsui, T.K.; Mori, E. Microglia support neural stem cell maintenance and growth. Biochem. Biophys. Res. Commun. 2018, 503, 1880–1884.
- Saraiva, C.; Barata-Antunes, S.; Santos, T.; Ferreiro, E.; Cristóvão, A.C.; Serra-Almeida, C.; Ferreira, R.; Bernardino, L. Histamine modulates hippocampal inflammation and neurogenesis in adult mice. Sci. Rep. 2019, 9, 8384.
- Aarum, J.; Sandberg, K.; Haeberlein, S.L.B.; Persson, M.A.A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl. Acad. Sci. USA 2003, 100, 15983–15988.
- Ribeiro Xavier, A.L.; Kress, B.T.; Goldman, S.A.; De Lacerda Menezes, J.R.; Nedergaard, M. A distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 2015, 35, 11848–11861.
- Doorn, K.J.; Brevé, J.J.; Drukarch, B.; Boddeke, H.W.; Huitinga, I.; Lucassen, P.J.; van Dam, A.M. Brain region-specific gene expression profiles in freshly isolated rat microglia. Front. Cell. Neurosci. 2015, 9, 84.
- Kreisel, T.; Wolf, B.; Keshet, E.; Licht, T. Unique role for dentate gyrus microglia in neuroblast survival and in VEGF-induced activation. Glia 2019, 67, 594–618.
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1145.
- Maekawa, M.; Takashima, N.; Arai, Y.; Nomura, T.; Inokuchi, K.; Yuasa, S.; Osumi, N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells 2005, 10, 1001–1014.
- Hodge, R.D.; Nelson, B.R.; Kahoud, R.J.; Yang, R.; Mussar, K.E.; Reiner, S.L.; Hevner, R.F. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J. Neurosci. 2012, 32, 6275–6287.
- Hodge, R.D.; Hevner, R.F. Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev. Neurobiol. 2011, 71, 680–689.
- Lavado, A.; Oliver, G. Prox1 expression patterns in the developing and adult murine brain. Dev. Dyn. 2007, 236, 518–524.
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H.H. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2+ Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell 2007, 1, 515–528.
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
01 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




