Jojoba is a widely used medicinal plant that is cultivated worldwide. Its seeds and oil have a long history of use in folklore to treat various ailments, such as skin and scalp disorders, superficial wounds, sore throat, obesity, and cancer; for improvement of liver functions, enhancement of immunity, and promotion of hair growth. Extensive studies on Jojoba oil showed a wide range of pharmacological applications, including antioxidant, anti-acne and antipsoriasis, anti-inflammatory, antifungal, antipyretic, analgesic, antimicrobial, and anti-hyperglycemia activities. In addition, Jojoba oil is widely used in the pharmaceutical industry, especially in cosmetics for topical, transdermal, and parenteral preparations. Jojoba oil also holds value in the industry as an anti-rodent, insecticides, lubricant, surfactant, and a source for the production of bioenergy. Jojoba oil is considered among the top-ranked oils due to its wax, which constitutes about 98% (mainly wax esters, few free fatty acids, alcohols, and hydrocarbons). In addition, sterols and vitamins with few triglyceride esters, flavonoids, phenolic and cyanogenic compounds are also present.
1. Introduction
The plant kingdom continues to hold considerable importance in our daily life. In addition to supplying humanity with food, it is considered as a potential source of thousands of novel materials such as fragrances, flavoring agents, dyes, fibers, beverages, building materials, heavy metal chelators, and many useful compounds of great therapeutic value.
Early studies of plants helped humankind make use of local flora for healing ailments. These studies have continued until now to seek out new agents for the treatment of various diseases. Recent investigations regarding plants with centuries of use in folk medicine have generated a great deal of information about the biologically active chemical components responsible for many claimed medicinal effects. As a result of thorough research involving the isolation and structure characterization techniques, many lead compounds, and prototypes from natural products have assumed reputable roles in medicine. Despite the huge number of synthetic and semisynthetic drugs, the most valuable medicinal agents still in use are obtained from medicinal plants.
There is high consumption of natural resources due to the increasing population. The resultant demand for green energy amidst fossil fuel shortages has rekindled interest in Jojoba oil (
Simmondsia chinensis (Link) Schneider). Jojoba oil is the only unsaturated liquid wax readily extractable in large quantities from plant sources (≈52% of the total seed weight), which shows a high structure similarity with the sperm whale oil. This similarity has increased the interest in Jojoba oil as a replacement for sperm whale oil (spermaceti wax) since the 1970s
[1].
Simmondsia chinensis (Link) Schneider is native to North and Central American deserts but cultivated worldwide in Chile, Egypt, and Argentina
[2]. Jojoba was widely used by Native Americans in the Sonora desert (California) as a foodstuff in the form of cooked fruits and in oil form as a therapeutic for multiple ailments: cancer therapy, liver and kidney disorders, obesity, parturition, sore throat, superficial wound healing, warts, psoriasis, acne, sunburn, and treatment of poison ivy exposure
[3][4][5][6]. Jojoba oil is widely used in the pharmaceutical industry, especially in cosmetics, to restore the ordinary health of hair and skin. The leaf extract, combined with extracts from other plants, also acts as anti-inflammatory agents to treat sensitive skin stress
[7]. Jojoba cosmetic products currently on the market include the following: bath oil, body oil, cleansing creams, cleansing pads, cleansing scrubs, nourishing facial cream, facial oil, hair conditioner, hair oil, makeup remover, and shaving cream
[8][9][10][11].
In addition, the oil has many industrial applications that include an extreme temperature/extreme pressure lubricant in the form of sulfurized oil, which can bear high temperature and pressure without changing its viscosity
[6][12]. Other industrial uses include the extraction and separation of isotopes such as Uranium (VI), Thorium (IV), and Plutonium (IV); in the leather industry as a fat liquor with good tanning properties
[5]; as a surfactant, fire retardant, lamp oil, candle wax, polishes
[13], and antifoaming agents in isolation of penicillin and tetracycline
[9].
An updated and in-depth review about jojoba oil chemistry, its pharmaceutical and industrial uses, and toxicity was conducted and is presented in this work to supplement the lack of comprehensive reviews covering the plant since the 1990s. The keywords jojoba, Simmondsia, chemistry, pharmaceutical preparations, emulgels, nanoparticles, toxicity, and biological activity were used in many combinations to search Scifinder®, PubMed®, Web of Science ® starting 1990 until 2021. English language was used as the only filter.
2. Common Names and Botanical Characteristics
The word jojoba, pronounced “ho-ho-ba”, is a distortion of the native Papago Indian word “howhowi”. Jojoba is known by many other names such as bucknut, coffee nut, goatnut, pignut, nutpush, goatberry, sheepnut, and lemon leaf
[14]. The seeds of the jojoba plant are dark brown, akin to large coffee beans.
Plants of the order Euphorbiales are usually herbs, shrubs, or sometimes trees
[15]. They are widely distributed globally, especially in temperate, subtropical, and tropical regions
[16]. They have frequently unisexual (rarely bisexual) hypogynous flowers, which are generally regular with a single whorl of a green perianth. The stamens are equal in number to perianth leaves or numerous. The pistil is composed of three carpels forming a trilocular ovary, with each chamber containing one or two anatropous, pendulous ovules in the inner angle with ventral or dorsal raphe
[17]. Simmondsiaceae is a small family of one genus,
Simmondsia, which is abundant in Southern Arizona, Sonora, and Baja California. Plants that belong to “Simmondsiaceae” are mostly woody branched shrubs that reach 2–4 m in height
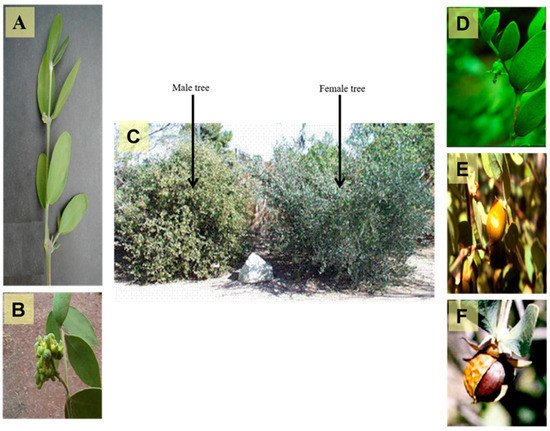
[4][5][6]. A photograph of male and female trees, flowers, and seeds of
Simmondsia chinensis are displayed in .
Figure 1. A photograph showing different organs of Simmondsia chinensis, (A) Branch of the plant (X 0.8), (B) Male flowers (X 1.0), (C) Old male and female trees (X 0.02), (D) Female flower (X 0.5), (E) Ripe fruit (X 0.5) and (F) Seed (X 0.8) (Photographer Eng. Nabil Elmougi, the jojoba farms of The Egyptian Natural Oil Company, Ismailia Desert Road, Egypt).
3. Chemical Constituents
Jojoba oil is composed of almost 98% pure waxes (mainly wax esters, few free fatty acids, alcohols, and hydrocarbons), sterols, and vitamins with few triglyceride esters, so it is widely known as liquid wax rather than oil or fat
[18].
3.1. Jojoba Wax
Investigation of the different organs of the jojoba plant for the presence of the wax revealed that the seeds contain most of the wax content in the plant (almost 50–52% of the seed weight)
[5]. Jojoba wax is composed mainly of esters and, to a lesser extent, free acids, free alcohols, and hydrocarbons
[4]. Esters are composed by the association of long straight-chain fatty acids with long straight-chain or higher molecular weight monohydric alcohols, C20 and C22; both the acids and alcohols are cis-monounsaturated at the (ω-9) position. Small triglyceride esters are also present
[19][20][21][22].
3.1.1. Wax Esters
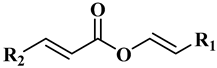
The main components of the wax esters that have been isolated and previously identified are docosenyl eicosenoate “erucyl jojobenoate” (1), eicosenyl eicosenoate “jojobenyl jojobenoate” (2), eicosenyl docosenoate “jojobenyl erucate” (3), docosenyl docosenoate (4), eicosenyl oleate (5), and docosenyl oleate (6) ()
[23]. Many other wax esters and free fatty alcohols and acids components are present in small quantities
[19][24].
Table 1. Chemical structures for the most abundant wax ester components in jojoba wax.
 |
|
| R1 = C20H41, R2 = C17 H35 |
Docosenyl eicosenoate (1) |
| R1 = C18H37, R2 = C17 H35 |
Eicosenyl eicosenoate (2) |
| R1 = C18H37, R2 = C19 H39 |
Eicosenyl docosenoate (3) |
| R1 = C16H33, R2 = C19 H39 |
Docosenyl docosenoate (4) |
| R1 = C18H37, R2 = C17 H33 (C9) |
Eicosenyl oleate (5) |
| R1 = C20H41, R2 = C17 H33 (C9) |
Docosenyl oleate (6) |
It was initially thought that jojoba wax esters were made up of random combinations of alcohols and acids until Miwa conducted a study on these combinations
[13]. He showed a significant difference between the observed results and those calculated by a random association of acids and alcohols. For instance, it was observed that (acid/alcohol, % experimental (% random)): (C20:1/C’20:1, 28.0% (31.8%)), (C20:1/C’22:1, 10.3% (5.7%)), (C22:1/C’20:1, 41.4% (32.0%)), (C22:1/C’22:1, 1.9% (5.7%)) indicating that eicosenyl docosenoate ester is preferably biosynthesized by the association of eicosenoic acid and docosenol. These combinations demonstrate that plants favor specific associations, which correspond to their genome. From an analytical point of view, this observation constitutes a valuable tool for detecting adulterated oil and good discrimination between natural Jojoba wax and its synthetic substitutes. In the latter case, associations between fatty acids and alcohols are governed by thermodynamic rules, and random results would be observed.
3.1.2. Free Fatty Acids and Alcohols
It was reported that the natural oil contains small quantities of free fatty acids (0.96%) and free alcohols (1.11%), as seen in ()
[13].
Table 2. The composition of free fatty alcohols and fatty acids derived from jojoba oil.
| Alcohols |
(%) |
Acids |
(%) |
| Tetradecanol |
trace |
Dodecanoic |
trace |
| Hexadecanol |
0.1 |
Tetradecanoic |
trace |
| Heptadec-8-enol |
trace |
Pentadecanoic |
trace |
| Octadecanol |
0.2 |
Hexadecanoic |
1.2 |
| Octadec-9-enol |
0.7 |
Hexadec-7-onoic |
0.1 |
| Octadec-11-enol |
0.4 |
Hexadec-9-enoic |
0.2 |
| Eicosanol |
trace |
Heptadecenoic |
trace |
| Eicos-11-enol |
43.8 |
Octadecanoic |
0.1 |
| Hecos-12-enol |
trace |
Octadec-9-enoic |
10.1 |
| Docosanol |
1.0 |
Octadec-11-enoic |
1.1 |
| Docos-12-enol |
44.9 |
Octadecadienoic |
0.1 |
| Tetracos-15-enol |
8.9 |
Octadecatrienoic |
trace |
| Hexacosenol |
trace |
Nonadecenoic |
trace |
| |
|
Eicosanoic |
0.1 |
| |
|
Eicos-l1-enoic |
71.3 |
| |
|
Eicosadienoic |
trace |
| |
|
Docosanoic |
0.2 |
| |
|
Docos-13-enoic |
13.6 |
| |
|
Tricosenoic |
trace |
| |
|
Tetracosenoic |
trace |
| |
|
Tetracos-15-enoic |
1.3 |
3.2. Sterols
There are many reports concerning the sterol content of jojoba oil
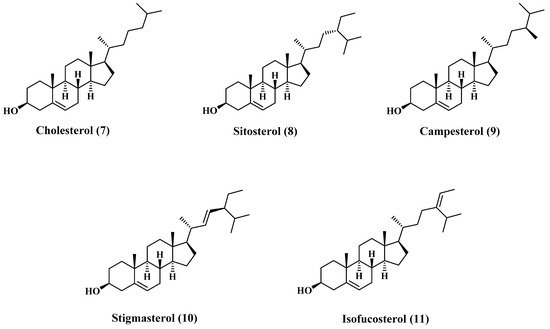
[5][19]. The major content of the sterols fraction is cholesterol (7), β-Sitosterol (8), campesterol (9), stigmasterol (10), and isofucosterol (11). Most of these sterols are sketched in , and the composition is tabulated in
[22].
Figure 2. Structures of major sterols content of jojoba oil.
Table 3. The average percentage of sterols content in jojoba oil.
| Sterol |
Sterol Fraction (%) |
Total Wax (mg/kg Seed) |
| Unidentified |
0.4 |
16 |
| Stigmasta-5,25-dien-3β-ol |
0.6 |
24 |
| Fucosterol |
0.6 |
24 |
| Isofucosterol |
4.1 |
163 |
| Cholesterol |
0.8 |
32 |
| Stigmasterol |
6.7 |
266 |
| Campesterol |
16.9 |
672 |
| Sitosterol |
69.9 |
2780 |
3.3. Flavonoids, Phenolic, and Cyanogenic Compounds
Although phenolic compounds are the most common secondary metabolites distributed in nature, they are only present in small quantities in jojoba, as reported in
[25]. Ten flavonoids have been identified as quercetin (12), quercetin 3′methyl ether (isorhamnetin), quercetin 3-methyl ether (14), quercetin 3,3′-dimethyl ether (15), isorhamnetin 3-
O-glucoside (16), quercetin 3-O-glucoside (17), typhaneoside (18), isorhamnetin 3-O-rutinoside (19), quercetin 3-
O-rutinoside (20). Some lignans are also present: (+)-lyoniresinol 4,4′-bis-
O-β-
d-glucopyranoside (21), salvadoraside (22), and eleutheroside E (23)
[26]. Simmondisin (24), simmonosides A (25), simmonosides B (26), and 4, 5-dimethyl-4-
O-alpha-
d-glucopyranosylsimmondsin (27) are the main cyanogenic glycosides
[20][21][27].
3.4. Fat-Soluble Vitamins
Vitamin D and its derivatives
viz. α, γ, and δ tocopherol were isolated and quantitatively estimated in the oil where γ-tocopherol makes up approximately 79% of these compounds. Other fat-soluble vitamins such as vitamin A are also found
[22].
4. Physical Characters of the Oil
Crude jojoba oil obtained directly by either cold expression or solvent extraction of the seeds, without any modifications, yields an oil with a golden or light yellow color. It has a pleasant, slightly nutty taste
[6]. The thermal and oxidative stabilities of the oil are high; therefore, the oil shows high resistance toward rancidity due to the presence of natural antioxidants (α, γ, and δ tocopherol)
[6]. Refined or bleached oil, obtained by passing the natural oil over activated charcoal and treating with caustic alkali substances, is nearly white with low oxidative stability due to subsequent removal of the antioxidants. The thermal stability of both natural and bleached forms is high, which is indicated by a high flash point reaching 295 °C
[28]. The oil’s viscosity favors using the oil and/or its derivatives as an extreme temperature/pressure lubricant
[6][12]. Jojoba oil is soluble in common solvents such as benzene and chloroform. However, it is essentially immiscible in methanol. The solubility of jojoba in different organic solvents ()
[29] and other physical properties () are listed in many previous works
[4][5][6][13][29][30][31].
Table 5. Solubility characteristics of jojoba oil in common organic solvents at 15 °C a.
| Solvent |
mL of Solvent |
Observation b |
| Water |
5.0 |
I |
| 10.0 |
I |
| Acetic acid |
10.0 |
I |
| 40.0 |
I |
| 50.0 |
S |
| Methanol |
1.0 |
I |
| 10.0 |
I |
| 40.0 |
S |
| Ethanol |
1.0 |
I |
| 5.0 |
I |
| 20.0 |
S |
| t-Amyl Alcohol |
1.0 |
S |
| 1-Butanol |
1.0 |
S |
| Acetone |
1.0 |
I |
| 3.0 |
I |
| 8.0 |
I |
| Benzene |
1.0 |
I |
| Toulene |
1.0 |
I |
| Carbon Tetrachloride |
1.0 |
I |
| s-Tetrachlocthane |
1.0 |
I |
| Diethylether |
1.0 |
I |
| Tetrahydrofuran |
1.0 |
I |
| Hexane |
1.0 |
I |
| Cyclohexane |
1.0 |
I |
| Dimethylformamide |
1.0 |
I |
| 10.0 |
I |
| 30.0 |
S |
| Dimethylsulfoxide |
1.0 |
I |
| 5.0 |
I |
| 20.0 |
S |
| Acetonitrile |
1.0 |
I |
| 10.0 |
I |
| 30.0 |
S |
| Aniline |
2.0 |
S |
| m-Cresol |
2.0 |
S |
Table 6. Some physical properties of jojoba oil as reported in the literature
[31].
| Freezing point, °C |
10.6–7.0 |
| Melting point, °C |
6.8–7.0 |
| Boiling point at 757 mm under N2, °C |
389 |
| Heat of fusion by DSC, Cal/g |
21 |
| Refractive index at 25 °C |
1.465 |
| Dielectric constant, 27 °C |
2.680 |
| Specific conductivity, 27 °C, mho/cm |
8.86.10–13 |
| Specific gravity, 25/25 °C |
0.863 |
| MV-1 rotor in MY cup, cp |
35 |
| Plate and cone with PK-1, cp |
33 |
| Brookfield, spindle #1, 25 °C, cp |
37 |
| Cannon–Fenske, 25 °C, cp |
50 |
| Cannon–Fenske, 100 °C centistokes |
27 |
| Smoke point, °C |
195 |
| Flash point, °C |
295 |
| Fire point, °C |
338 |
| Iodine value |
82 |
| Saponification value |
92 |
| Acid value |
<2 |
| Acetyl value |
2 |
| Unsaponifiable matter, % |
51 |
| Total acids, % |
52 |
| Iodine value of alcohols |
77 |
| Iodine value of acids |
<76 |
| Average molecular weight of wax esters |
606 |
5. Chemical Properties of the Oil
Jojoba molecules contain two double bonds at ω-9 positions in both alcohol and acid sides, which are separated by an ester bond. While in typical plant oils, double bonds are usually close to each other; in jojoba molecules, they are far apart and uneven from the center. These three active sites have been proven to be the source of many intermediates and final products with different physical and chemical characters. These derivatives are described with particular reference to the reaction that leads to the formation of a wide potential of industrial and pharmaceutical applications: the production of semisoft waxes by geometrical isomerization in the manufacture of suppository bases, production of hard waxes by hydrogenation in the manufacture of candles, production of additives by sulfurization for high-pressure/high-temperature lubricants, production of selective exectrants for the nuclear industry by phosphonation of chemically bonded jojoba oil
[5][32][33].
These chemical modifications provide a wide range of polymers with diverse properties that could serve as good candidates in industrial application, especially those related to the polyhydroxyurethanes polymers that will be discussed in detail in
Section 8.1.
Shani continued his basic research on the possible reaction schemes at the double bond. All-trans jojoba oil was prepared by the straight chemical route, involving the anti-addition of bromine or chlorine to the double bonds followed by displacement and elimination of the halogens with Na I. All-
trans jojoba was obtained at a yield greater than 75% and had a melting point of 52–54 °C. Similar to those obtained from natural liquid oil, a series of products were prepared from the semisolid all-
trans jojoba under the same conditions used for the natural liquid oil
[34][35].
Thorough investigations of the all-trans derivatives and their physical and chemical characteristics revealed that the all-trans derivatives of jojoba oil had essentially the same melting points as those of the cis configuration. Based on these observations, Shani concluded that the polar groups played a more significant role than the geometrical configuration of the double bonds in determining the strength of the packing of the molecules in the solid phase.
5.1. Cis/Trans Isomerization
Unsaturated fatty materials can be converted into solid materials by geometrical isomerization of the double bonds. The
trans isomer of jojoba is thermodynamically more stable than the
cis form. Moreover, it has a substantially higher melting point, and its soaps have superior wetting and detergency properties. The reaction has never had any commercial significance, which is most likely because the same results can be achieved by partial hydrogenation with the additional advantages of higher oxidation stability. This phenomenon could expand the uses of this isomerized material, especially as a suppository base in the pharmaceutical industry due to its natural creamy appearance coupled with a melting point close to human body temperature
[6].
Wisniak and Alfandary were the first to report on the geometrical isomerization of jojoba oil with selenium and NO
2 catalysts under a wide range of conditions. Melting points of the resultant product varied between 36 and 42 °C. Proper adjustment of the operating conditions could, if necessary, allow the preparation of a material with a melting point close to average human body temperature
[36]. Later, Galun and coworkers described the thermal and photosensitized isomerization of jojoba oil. The absorption of light at wavelengths of 366 nm or more via the allowance of sensitizers enables the acquisition of the isomerized form of jojoba oil. However, this is dependent on the fact that the
cis isomer can transform into the trans form if heated to a temperature sufficiently high and that the double bonds present in jojoba oil absorb light of wavelengths below 200 nm as a result
[37][38].
5.2. Hydrogenation
Hydrogenation is a standard technique for improving the properties of vegetable and animal oils. In addition, it increases the softening and melting points of the fats and improves their color, odor, and stability. The reaction involves the chemical addition of hydrogen to the unsaturated carbon-to-carbon double bonds in fatty alcohol or the fatty acid molecule. This addition occurs by mixing the heated oil and hydrogen in the presence of nickel as a catalyst, at pressures in the order of 0–120 psi, and in hydrogenation conditions widely used in industrial purposes
[5]. Total hydrogenation of the oil produces highly lustrous, pearly white crystalline laminae that are very hard. Solid wax has been suggested as a potential ingredient in polish waxes, carbon paper, waxing of fruit, and candles component
[5].
In 1959, a comparison was made by Knoepfler et al. between the hydrogenation characteristics of jojoba oil obtained by extracting the oil with solvents and those obtained by the cold-hydraulic pressing of the jojoba seeds. The results revealed no significant difference between both hydrogenated forms, except in the melting point. Those prepared from cold-hydraulic pressing have a melting point of 67–68 °C, while those prepared from oil extracted by solvents have a higher melting point of 74–76 °C
[32]. Wisniak and Holin have studied the hydrogenation of jojoba oil under a wide range of operating conditions and reaction kinetics in the preparation of different types of solid waxes and compared the characters of the hydrogenated jojoba oil with Beeswax and Carnauba wax. It was observed that jojoba oil is substantially better than Beeswax and relatively equal with Carnauba wax regarding hydrogenation
[39]. Simpson and Miwa have done an in-depth X-ray diffraction study of hydrogenated jojoba oil to determine fatty acid and alcohol chain conformation, unit cell, and angle of tilt of the chains
[40].
5.3. Halogenation
Halogenated fatty materials find extensive uses in preparing quaternary compounds, anti-rotting, flame proofing, and fungicide additives. In addition, brominated vegetable oils have long been used as weighting oil in carbonated beverages
[41]. In 1979, Wisniak and Alfandary conducted an extensive study of the chlorination and bromination of jojoba oil. Their main objective was to determine the kinetics of the reaction and evaluate the influence of the operating variables. The experimental results indicated that in the dark and the temperature range used (−15 to +5 °C), a direct addition to the double bond with essentially no substitution occurred. The rate of halogenation decreased with the increase in temperature
[42].
5.4. Sulfurization and Sulfur Halogenation
The sulfurization of fatty material with sulfur or other reagents containing sulfur and halogen yields various products with different physical and chemical properties. In general, when sulfur content is low (<5%), the products will be liquid and be used as additives. Increasing the sulfur content will increase the viscosity until a rubber-like mass is obtained.
In 1975, Gisser et al. conducted a deep study about the mechanical properties of sulfurized jojoba oil and sulfurized sperm oil. The results obtained revealed that there is no difference between both oils
[5]. Furthermore, sulfurized jojoba oil has additional advantageous properties regarding its appearance and high viscosity; the same result was obtained by Miwa et al.
[13].
5.5. Phosphonation
Dialkyl alkylphosphantes are stable organic phosphorus esters possessing unique properties and offer considerable potential for commercial exploitation. Thus, they have been recommended for use in many applications. As plasticizers, they hold great potential due to their superior stability and other unique characteristics compared to organic phosphates. They have been suggested as synthetic lubricants, additives to improve the extreme pressure properties of lubricants, functional fluids, oil, or fuel additives; pour-point depressants, pesticides, synergists, or carriers for pesticides and fertilizers, intermediates for the synthesis of corrosion inhibitors, and metal extractants. In general, several dialkyl alkylphosphantes are useful as flame-retardants, softeners, textile treating agents, and heat transfer media
[5]. Wisniak has reported preliminary experimental data on the phosphonation of jojoba oil with different dialkylphosphites, using tert-butyl perbenzoate as a radical generator. The average ester chain in jojoba oil contains two double bonds so that the final product may contain up to two atoms of phosphorus per chain
[33][42].
5.6. Oxidation, Epioxidation, and Ozonolysis
Jojoba oil shows good thermal stability up to a relatively high temperature. Generally, the cosmetic formulations containing jojoba oil have superior stability toward oxidation than other lipids used for this purpose. A comparative study of the relative oxidation stability of jojoba oil, sperm whale oil, carnauba wax esters, Limnanthes douglassi wax esters, and behenyl arachidate revealed that jojoba oil has high oxidative stability comparing all other oils
[43]. Kampf conducted an in-depth study of the accelerated oxidation of crude jojoba oil and bleached and stripped oils. He found that crude jojoba oil contains natural antioxidants that counted for the high oxidative stability of the natural oil. The removal of these antioxidants through bleaching or stripping of the oil leads to a sharp decline in the oxidative stability of the products
[5]. Epoxides of unsaturated glycerides and simple fatty acid esters are currently used as plasticizers and stabilizers for polyvinyl chloride plastics
[5].
Ozonolysis is an important technique for studying the structure of unsaturated compounds such as those present in jojoba oil. Ozonides in general, but particularly jojoba ozonides, are viable intermediates for many synthetic paths. Zabicky previously used ozone as a reagent to attain intermediates to synthesize different derivatives that are widely used for industrial purposes
[5].