
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daniel López Malo | + 3107 word(s) | 3107 | 2021-05-27 04:29:43 | | | |
| 2 | Vivi Li | Meta information modification | 3107 | 2021-05-28 03:45:32 | | | | |
| 3 | Conner Chen | Meta information modification | 3107 | 2021-08-04 11:18:27 | | | | |
| 4 | Conner Chen | Meta information modification | 3107 | 2021-09-22 02:33:30 | | |
Video Upload Options
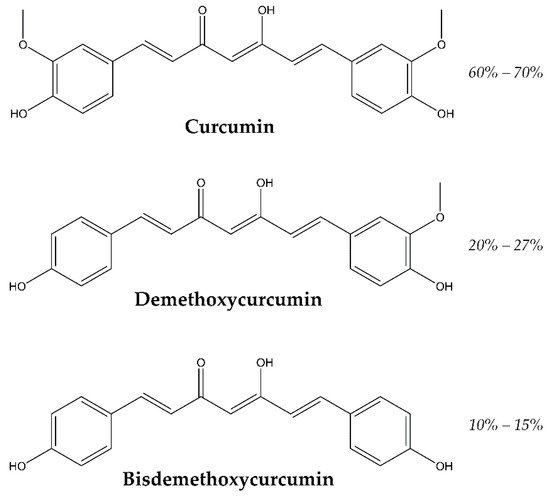
The retina is subjected to oxidative stress due to its high vascularization, long time light exposition and a high density of mitochondria. Oxidative stress can lead to pathological processes, like cell apoptosis, angiogenesis and inflammation ending in retinal pathologies. Curcumin, a major bioactive component obtained from the spice turmeric (Curcuma longa) rhizome has been used for centuries in Asian countries for cooking and for curing all kinds of diseases like dysentery, chest congestion and pain in general, due to its antioxidant effects. Curcumin prevents the formation of reactive oxygen species and so it is a good protective agent. Curcumin has shown also anti-inflammatory, and antitumor properties. Curcumin is a natural product, which can be a therapeutic option in a variety of retinal diseases due to its pleiotropic properties. Some drawbacks are its poor solubility, bioavailability and lack of stability at physiological conditions; which have been shown in curcumin skeptical publications.
1. Introduction
2. Diabetic Retinopathy (DR)
3. Eye Antitumor Activity (Retinal and Choroidal Tumors)
4. Curcumin Delivery
4.1. Encapsulation
4.2. Curcumin Analogues
References
- Levine, B.; Klionsky, D.J. Development by Self-Digestion: Molecular Mechanisms and Biological Functions of Autophagy. Dev. Cell 2004, 6, 463–477.
- Wright, A.F.; Chakarova, C.F.; Abd El-Aziz, M.M.; Bhattacharya, S.S. Photoreceptor degeneration: Genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010, 11, 273–284.
- Beatty, S.; Koh, H.-H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134.
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. 2013, 752, 153–171.
- Arnal, E.; Johnsen-Soriano, S.; Lopez-Malo, D.; Perez-Pastor, G.; Vidal-Gil, L.; Morillas, N.; Sancho-Pelluz, J.; Romero, F.; Barcia, J. Docosahexaenoic Acid Protects against High Glucose-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. React. Oxyg. Species 2016, 2, 298–307.
- Kamoshita, M.; Toda, E.; Osada, H.; Narimatsu, T.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci. Rep. 2016, 6, 30226.
- Arnal, E.; Miranda, M.; Johnsen-Soriano, S.; Alvarez-Nlting, R.; Daz-Llopis, M.; Araiz, J.; Cervera, E.; Bosch-Morell, F.; Romero, F.J. Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats. Curr. Eye Res. 2009, 34, 928–938.
- Di Marco, S.; Carnicelli, V.; Franceschini, N.; Di Paolo, M.; Piccardi, M.; Bisti, S.; Falsini, B. Saffron: A Multitask Neuroprotective Agent for Retinal Degenerative Diseases. Antioxidants 2019, 8, 224.
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 4110.
- Yang, Y.; Qin, Y.J.; Yip, Y.W.Y.; Chan, K.P.; Chu, K.O.; Chu, W.K.; Ng, T.K.; Pang, C.P.; Chan, S.O. Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep. 2016, 6, 29546.
- Martínez-Solís, I.; Acero, N.; Bosch-Morell, F.; Castillo, E.; González-Rosende, M.E.; Muñoz-Mingarro, D.; Ortega, T.; Sanahuja, M.A.; Villagrasa, V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Med. 2019.
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254.
- Liu, X.F.; Hao, J.L.; Xie, T.; Mukhtar, N.J.; Zhang, W.; Malik, T.H.; Lu, C.W.; Zhou, D.D. Curcumin, a potential therapeutic candidate for anterior segment eye diseases: A review. Front. Pharmacol. 2017, 8, 66.
- Peddada, K.V.; Brown, A.; Verma, V.; Nebbioso, M. Therapeutic potential of curcumin in major retinal pathologies. Int. Ophthalmol. 2019, 39, 725–734.
- Farajipour, H.; Rahimian, S.; Taghizadeh, M. Curcumin: A new candidate for retinal disease therapy? J. Cell. Biochem. 2019, 120, 6886–6893.
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 299–312.
- Uehara, S.; Yasuda, I.; Takeya, K.; Itokawa, H. Terpenoids and curcuminoids of the rhizoma of Curcuma xanthorrhiza Roxb. Yakugaku Zasshi 1992, 112, 817–823.
- Chignell, C.F.; Bilskj, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral And photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302.
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999, 121, 161–175.
- Agnihotri, N.; Mishra, P.C. Scavenging mechanism of curcumin toward the hydroxyl radical: A Theoretical study of reactions producing ferulic acid and vanillin. J. Phys. Chem. A 2011, 115, 14221–14232.
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986.
- Ciulla, T.A.; Amador, A.G.; Zinman, B. Diabetic retinopathy and diabetic macular edema: Pathophysiology, screening, and novel therapies. Diabetes Care 2003, 26, 2653–2664.
- Kowluru, R.A.; Chan, P.-S. Oxidative Stress and Diabetic Retinopathy. Exp. Diabetes Res. 2007, 2007, 43603.
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184.
- Chiu, J.; Xu, B.Y.; Chen, S.; Feng, B.; Chakrabarti, S. Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can. J. Physiol. Pharmacol. 2008, 86, 365–372.
- Kumar, P.A.; Haseeb, A.; Suryanarayana, P.; Ehtesham, N.Z.; Reddy, G.B. Elevated expression of αA—And αB-crystallins in streptozotocin-induced diabetic rat. Arch. Biochem. Biophys. 2005, 444, 77–83.
- Mrudula, T.; Suryanarayana, P.; Srinivas, P.N.B.S.; Reddy, G.B. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochem. Biophys. Res. Commun. 2007, 361, 528–532.
- Kowluru, R.A.; Kanwar, M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007, 4, 8.
- Rajpathak, S.N.; Gunter, M.J.; Wylie-rosett, J.; Ho, G.Y.F.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 28, 3–12.
- Platania, C.B.M.; Fidilio, A.; Lazzara, F.; Piazza, C.; Geraci, F.; Giurdanella, G.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. Retinal protection and distribution of curcumin in vitro and in vivo. Front. Pharmacol. 2018, 9, 670.
- Bucolo, C.; Drago, F.; Maisto, R.; Romano, G.L.; D’Agata, V.; Maugeri, G.; Giunta, S. Curcumin prevents high glucose damage in retinal pigment epithelial cells through ERK1/2-mediated activation of the Nrf2/HO-1 pathway. J. Cell. Physiol. 2019, 234, 17295–17304.
- Dimaras, H.; Corson, T.W. Retinoblastoma, the visible CNS tumor: A review. J. Neurosci. Res. 2019, 97, 29–44.
- Munier, F.L.; Beck-Popovic, M.; Chantada, G.L.; Cobrinik, D.; Kivelä, T.T.; Lohmann, D.; Maeder, P.; Moll, A.C.; Carcaboso, A.M.; Moulin, A.; et al. Conservative management of retinoblastoma: Challenging orthodoxy without compromising the state of metastatic grace. “Alive, with good vision and no comorbidity. Prog. Retin. Eye Res. 2019.
- Yu, X.; Zhong, J.; Yan, L.; Li, J.; Wang, H.; Wen, Y.; Zhao, Y. Curcumin exerts antitumor effects in retinoblastoma cells by regulating the JNK and p38 MAPK pathways. Int. J. Mol. Med. 2016, 38, 861–868.
- Li, Y.; Sun, W.; Han, N.; Zou, Y.; Yin, D. Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway. BMC Cancer 2018, 18, 1230.
- Karakawa, A.; Taoka, K.; Kaburaki, T.; Tanaka, R.; Shinozaki-Ushiku, A.; Hayashi, H.; Miyagi-Maeshima, A.; Nishimura, Y.; Uekusa, T.; Kojima, Y.; et al. Clinical features and outcomes of secondary intraocular lymphoma. Br. J. Haematol. 2018, 183, 668–671.
- Bar-Sela, G.; Epelbaum, R.; Schaffer, M. Curcumin as an Anti-Cancer Agent: Review of the Gap Between Basic and Clinical Applications. Curr. Med. Chem. 2009, 17, 190–197.
- Lu, H.-F.; Lai, K.-C.; Hsu, S.-C.; Lin, H.-J.; Yang, M.-D.; Chen, Y.-L.; Fan, M.-J.; Yang, J.-S.; Cheng, P.-Y.; Kuo, C.-L.; et al. Curcumin induces apoptosis through FAS and FADD, in caspase-3-dependent and -independent pathways in the N18 mouse-rat hybrid retina ganglion cells. Oncol. Rep. 2009, 22, 97–104.
- Lu, H.F.; Yang, J.S.; Lai, K.C.; Hsu, S.C.; Hsueh, S.C.; Chen, Y.L.; Chiang, J.H.; Lu, C.C.; Lo, C.; Yang, M.D.; et al. Curcumin-induced DNA damage and inhibited dna repair genes expressions in mouse-rat hybrid retina neuroblastoma cells ganglion cells (n18). Neurochem. Res. 2009, 34, 1491–1497.
- Lin, H.-J.; Su, C.-C.; Lu, H.-F.; Yang, J.-S.; Hsu, S.-C.; Ip, S.-W.; Wu, J.-J.; Li, Y.-C.; Ho, C.-C.; Wu, C.-C.; et al. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol. Rep. 2010, 23, 665–670.
- Burugula, B.; Ganesh, B.S.; Chintala, S.K. Curcumin attenuates staurosporine-mediated death of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4263–4273.
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818.
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Controlled release of curcumin from thiolated starch-coated iron oxide magnetic nanoparticles: An in vitro evaluation. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 349–358.
- Sadeghzadeh, H.; Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Dariushnejad, H.; Sanjarian, F.; Zarghami, N. The Effects of Nanoencapsulated Curcumin-Fe3O4 on Proliferation and hTERT Gene Expression in Lung Cancer Cells. Anticancer Agents Med. Chem. 2017, 17, 1363–1373.
- Wang, J.; Wang, F.; Li, F.; Zhang, W.; Shen, Y.; Zhou, D.; Guo, S. A multifunctional poly(curcumin) nanomedicine for dual-modal targeted delivery, intracellular responsive release, dual-drug treatment and imaging of multidrug resistant cancer cells. J. Mater. Chem. B 2016, 4, 2954–2962.
- Lachowicz, D.; Szpak, A.; Malek-Zietek, K.E.; Kepczynski, M.; Muller, R.N.; Laurent, S.; Nowakowska, M.; Zapotoczny, S. Biocompatible and fluorescent superparamagnetic iron oxide nanoparticles with superior magnetic properties coated with charged polysaccharide derivatives. Colloids Surf. B Biointerfaces 2017, 150, 402–407.
- Hou, L.; Shi, Y.; Jiang, G.; Liu, W.; Han, H.; Feng, Q.; Ren, J.; Yuan, Y.; Wang, Y.; Shi, J.; et al. Smart nanocomposite hydrogels based on azo crosslinked graphene oxide for oral colon-specific drug delivery. Nanotechnology 2016, 27, 315105.
- Some, S.; Gwon, A.R.; Hwang, E.; Bahn, G.H.; Yoon, Y.; Kim, Y.; Kim, S.H.; Bak, S.; Yang, J.; Jo, D.G.; et al. Cancer therapy using ultrahigh hydrophobic drug-loaded graphene derivatives. Sci. Rep. 2014, 4, 6314.
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of curcumin in cyclodextrin-metal organic frameworks: Dissociation of loaded CD-MOFs enhances stability of curcumin. Food Chem. 2016, 212, 485–494.
- Danafar, H.; Davaran, S.; Rostamizadeh, K.; Valizadeh, H.; Hamidi, M. Biodegradable m-PEG/PCL core-shell micelles: Preparation and characterization as a sustained release formulation for curcumin. Adv. Pharm. Bull. 2014, 4, 501–510.
- Jourghanian, P.; Ghaffari, S.; Ardjmand, M.; Haghighat, S.; Mohammadnejad, M. Sustained release curcumin loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 17–21.
- Zs. Nagy, N.; Varga, Z.; Mihály, J.; Kasza, G.; Iván, B. Kiss Highly efficient encapsulation of curcumin into and pH-controlled drug release from poly(ε-caprolactone) nanoparticles stabilized with a novel amphiphilic hyperbranched polyglycerol. Express Polym. Lett. 2020, 14, 90–101.
- Kumari, P.; Muddineti, O.S.; Rompicharla, S.V.K.; Ghanta, P.; Adithya, K.B.B.N.; Ghosh, B.; Biswas, S. Cholesterol-conjugated poly(D, L-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017, 24, 209–223.
- Kalani, A.; Chaturvedi, P.; Kamat, P.K.; Maldonado, C.; Bauer, P.; Joshua, I.G.; Tyagi, S.C.; Tyagi, N. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int. J. Biochem. Cell Biol. 2016, 79, 360–369.
- Guerzoni, L.P.B.; Nicolas, V.; Angelova, A. In Vitro Modulation of TrkB Receptor Signaling upon Sequential Delivery of Curcumin-DHA Loaded Carriers Towards Promoting Neuronal Survival. Pharm. Res. 2017, 34, 492–505.
- Granata, G.; Paterniti, I.; Geraci, C.; Cunsolo, F.; Esposito, E.; Cordaro, M.; Blanco, A.R.; Cuzzocrea, S.; Consoli, G.M.L. Potential Eye Drop Based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability, and Anti-Inflammatory Effect. Mol. Pharm. 2017, 14, 1610–1622.
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 11066.
- Kim, D.; Maharjan, P.; Jin, M.; Park, T.; Maharjan, A.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential Albumin-Based Antioxidant Nanoformulations for Ocular Protection against Oxidative Stress. Pharmaceutics 2019, 11, 297.
- Maharjan, P.; Jin, M.; Kim, D.; Yang, J.W.; Maharjan, A.; Shin, M.C.; Cho, K.H.; Kim, M.S.; Min, K.A. Evaluation of epithelial transport and oxidative stress protection of nanoengineered curcumin derivative-cyclodextrin formulation for ocular delivery. Arch. Pharm. Res. 2019, 42, 909–925.
- Cheng, Y.H.; Ko, Y.C.; Chang, Y.F.; Huang, S.H.; Liu, C.J. ling Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2019, 179, 179–187.
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin analogues and their hybrid molecules as multifunctional drugs. Eur. J. Med. Chem. 2019, 182, 111631.
- Pittalà, V.; Salerno, L.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Drago, F.; Bucolo, C.; Foresti, R. Effects of Novel Nitric Oxide-Releasing Molecules against Oxidative Stress on Retinal Pigmented Epithelial Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1420892.
- Wang, J.; Zhou, J.; He, H.; Wu, D.; Du, X.; Xu, B.; Xiong, T.; Li, X. Enzymatic formation of curcumin in vitro and in vivo. Nano Res. 2018, 11, 3453–3461.
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367.




