Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hossein Zargarnezhad | + 2838 word(s) | 2838 | 2021-05-26 05:38:28 | | | |
| 2 | Peter Tang | Meta information modification | 2838 | 2021-05-27 03:52:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zargarnezhad, H. Polymeric Pipeline Coatings Time-Dependent Performance. Encyclopedia. Available online: https://encyclopedia.pub/entry/10139 (accessed on 13 January 2026).
Zargarnezhad H. Polymeric Pipeline Coatings Time-Dependent Performance. Encyclopedia. Available at: https://encyclopedia.pub/entry/10139. Accessed January 13, 2026.
Zargarnezhad, Hossein. "Polymeric Pipeline Coatings Time-Dependent Performance" Encyclopedia, https://encyclopedia.pub/entry/10139 (accessed January 13, 2026).
Zargarnezhad, H. (2021, May 26). Polymeric Pipeline Coatings Time-Dependent Performance. In Encyclopedia. https://encyclopedia.pub/entry/10139
Zargarnezhad, Hossein. "Polymeric Pipeline Coatings Time-Dependent Performance." Encyclopedia. Web. 26 May, 2021.
Copy Citation
The barrier performance of organic coatings is a direct function of mass transport and long-term stability of the polymeric structure. A predictive assessment of the protective coating cannot be conducted a priori of degradation effects on transport. Epoxy-based powder coatings are an attractive class of coatings for pipelines and other structures because application processing times are low and residual stresses between polymer layers are reduced.
powder-based coatings
glassy epoxy
mass transfer properties
wet-state diffusion
permeability
coating degradation
1. Introduction
Many coating technologies have been developed to fulfill industrial demands for protective barriers for steel structures [1]. However, coating technologies face increasing challenges posed by high or low operating temperatures as well as new requirements for increased longevity and abrasion resistance [2]. Epoxy-based coatings are commonly used for oil and gas pipelines and other steel structures [3]. In addition to good performance for corrosion protection and resistance to weather, and humidity [4], epoxy-based powder coatings have a significantly shorter curing time relative to liquid and thermoplastic coatings—they reduce processing time while providing optimal anticorrosion performance [5]. The few cases of early powder coating failure documented in the literature are mostly due to inappropriate substrate pretreatment, low application temperature, improper curing, or improper chemistry in the formulation [6].
The development of an organic coating requires compatibility with specific environmental regulations and safety concerns [7]; the coating’s molecular structure is modified in some cases to meet new expectations [8][9][10]. For instance, the peripheral polar functional groups in the epoxy act as adsorption sites for inhibitors (e.g., amines, thiols, and alcohol-based epoxy resins), which enhances its anticorrosive effects in aqueous media when applied as a coating [11]. In addition to the main macromolecular compound, organic and inorganic additive components can be used to improve the anticorrosive performance of the coating [12]. In general, functional components in a coating formulation serve different purposes: solvent to control film adhesion/formation; organic or polymeric binders to provide barrier properties; dispersed pigments/functional fillers to enhance mass transport properties and UV resistance; fillers to provide uniformity, and other additives to inhibit substrate corrosion [4][13]. There are many proposed new coating systems for petroleum-based products and, since climate change is a serious concern, recent trends in alternative fuels suggest that there is a necessity to increase the bio-based material content in coatings [14][15][16]. However, the pipeline industry continues to use epoxy-based coatings. Some functional fillers such as needle-shaped amorphous wollastonite are used to increase flexural modulus and lower the thermal expansion and shrinkage of the final coating [17][18]. Microencapsulated agents―also known as self-healing components―may also be used as additives. These provide a polymer mending property in case of mechanical damage to the coating and may effectively protect the coated steel surface from corrosion [19][20].
Environmental parameters such as humidity, temperature, operating pressure, and ageing can individually or in combination induce various complex mass transport scenarios [21][22]. The interactions between the varied components of the coating and the eventual service conditions are central to the long-term performance of the coating. The mass transport properties of the coating provide a metric for this interplay. Effects of the coating’s structural attributes—e.g., its polarity, chain stiffness, and inertness to the penetrant—on the extent of permeation are discussed in the literature [23], however, data on transport rates of various species and their competitive permeation through coatings are scant.
Fusion-bonded epoxy (FBE) is a thermoset polymer with excellent long-term adhesion performance at moderate temperatures (e.g., 65 °C) [19] and is often preferred for both internal linings and external coatings on pipelines [24][25]. Outside a few reports on individual field-applied coatings, there are no standard data for the mass transfer properties of FBE [26][27], possibly because of the specificity of the diverse FBE formulations to various applications [28]. In severe environments, additional polyolefin layers may be applied over an FBE primer to reinforce the protection against corrosive species [29][30][31]. High-Performance Powder Coating (HPPC) is a monolithic coating structure with efficient interlayer compatibility, and is qualified for service under aggressive environments [32][33]. Although excellent results have been reported, demands for aggressive working conditions (e.g., wet-state applications, high temperatures, oxygen, and salts) may pose new challenges for these protection systems [34]. By focusing on these technologies as exemplars of single- and multi-layered coatings in the present study, we outline challenges involved in gaining a predictive approach for analysis of a given coating’s long-term performance.
2. Mass Transport through Coating Materials
Mass transfer characteristics of coatings have received less attention than post-disbondment events, especially for pipeline materials in which long-term integrity is of major interest. Failure analysis of pipeline corrosion has shown that the most common forms of coating defect are holidays and cathodic disbondment (CD) [37][38], and thus, significant progress has been made to enhance the durability of organic coatings against these issues. However, environmental effects on operating pipeline coatings, such as moisture and temperature, are understudied or unexplored. Among components present in the coating formulation, binders are expected to facilitate water and oxygen transmission [39] and, consequently, could contribute to matrix deterioration. Permeabilities of a coating system against water, oxygen, and ionic species are fundamental attributes necessary to identify rate-determining steps of corrosion of the underlying steel substrate [23]. According to the solution-diffusion model, transmission of permeant molecules through polymeric coating films (expressed as a permeability coefficient) occurs in a three-step process [40]:
-
Dissolution in the polymer (determined by the solubility coefficient) from the exposed side
-
Diffusion from higher to lower concentration/pressure (determined by diffusion coefficient)
-
Desorption from the other side of the polymer film
Permeability is thus a function of solubility in the coating and diffusivity through the coating, either of which may dominate depending upon interactions between the polymer and the permeant. Generally, diffusivity dominates in gaseous permeation, unless the gas is easily condensable (e.g., CO2) or is water vapor [41]. When a coating system is required to prevent simultaneous ingress of gases and liquid water, data from individual species permeability tests (e.g., oxygen permeability in the absence of water vapor) may not adequately represent barrier properties.
3. The Long-Term Stability of FBE and Its Effects on Mass Transport
Adhesion is a key characteristic in coating degradation. Adhesion failure, and the resultant underlying corrosion, facilitate the flux of aggressive species across the coating. However, unless a major defect (e.g., microcracking) evolves from internal stresses in the coating membrane, barrier protection remains governed by transport processes through the coating. During the initial coating process, when a liquid FBE film flows onto a pretreated steel surface (i.e., cleaned with sandblasting and acid washed), hydroxyl groups along the molecular chain enable the coating structure to form robust anchor points. It is also necessary that the terminal epoxy groups adequately crosslink in the profile of the film to minimize internal stresses [42]. The resin molecule in FBE contains a three-membered ring, oxirane, which is highly reactive when curing takes place at high temperature—i.e., 180 °C to 200 °C for Low Application Temperature (LAT) FBE and up to 250 °C with standard and high Tg FBE [43][44]. Apart from its processing advantages, this layer does not require the solvent to keep the binder and filler parts in a liquid suspension form. The final non-crystalline solid remains a glassy polymer at the operating temperature (e.g., 65 °C). Modifications of FBE molecular network (e.g., bromination of the phenyl functional group) result in Tg increase, and consequently, improvements in adhesion and CD performance of the final coating [45]. Dispersion of filler materials such as carbon black in the powder blend can also enhance barrier properties as a result of decreasing porosity and chain segment motions [46]. However, one must note that a higher amount of filler particles increases the tendency for agglomeration, and thus, deteriorates barrier properties. In addition, the size and shape of the filler particles play important roles in erosion resistance [47][48][49].
In pipeline coating systems, myriad environmental and design parameters can exert a variety of forces to drive mass transport through the coating. In the early stages of degradation, Fickian diffusion takes place as a result of the water concentration gradient (i.e., vapor pressure difference or in the case of offshore areas, hydrostatic pressure) across the polymer coating. Likewise, the presence of salt in the vicinity of the coating (such as sodium and chloride in seawater or soil) results in transport of ionic species through the system. Measurements can become intricate when an applied potential is introduced to the system: the external electron source—e.g., cathodic protection (CP)—supports the ionic flow through electrostatic forces [50]. In addition, the presence of environmental CO2 (as in soil) makes the pipeline steel susceptible to stress corrosion cracking; this type of corrosion attack is most likely to occur at coating imperfection areas [51]. However, the high tendency of condensable gases like CO2 to sorb into microvoids of glassy polymeric coatings can facilitate their gradual gas permeation [52][53]. Relevant data such as CO2 transport through coatings, especially for hydrated epoxy, are missing in the literature.
The FBE coating may begin to degrade prior to the service life of the pipe due to storage conditions, for example, via ultraviolet (UV) exposure during stockpiling in the field [54]. Physical ageing of glassy polymers leads to rapid deterioration of transport properties, usually accompanied by an increase in selectivity of the polymer against a gas mixture [55]. Studies on gas separation membranes have shown that thinner coating layers physically age more rapidly [56]; because the standard application thickness of FBE coatings on pipelines is thin (i.e., 350–500 µm), they fall into this accelerated ageing category. Latino et al. [57] found that hydrothermal ageing of FBE at 85 °C for three to seven months results in a minimum for Tg. This then results in an increase of the electrical conductivity of the FBE, and despite further water uptake by the coating, Tg does not subsequently drop. FTIR analysis of their FBE samples (Figure 1) showed that, in addition to O–H stretching (peaks between 3610 cm−1 and 3210 cm−1) from water uptake, new vibrations occur in other chemical bonds in the FBE structure (e.g., water reaction with carbon double-bond groups at 900 cm−1) [57]. On the other hand, they observed a signal increase at 1608 and 1732 cm−1 for aromatic rings and esters stretches, respectively, which may reflect chain scission of the epoxy backbone component and subsequent leaching of smaller molecular weight products towards the surface. This effect was previously reported for epoxy structures: at the maximum water sorption, the diffusion coefficient of oxygen decreases, suggesting chain scission and loss of volatile products in the epoxy. However, it has also been observed that this scission is followed by the formation of the new cross-links [58][59]. The Tg of the coating may partially be recovered upon removal of water through high vacuum drying, which can result in limited reversible cross-linking [60].
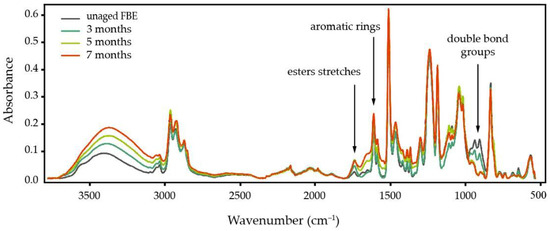
Figure 1. Degradation of FBE molecular structure caused by water sorption: after three months hydrothermal ageing, water can disrupt epoxy double bonds and cause chain scission for the backbone. Copyright 2019. Reproduced from [57] with permission from Elsevier Ltd.
4. Multi-Layer Coating Systems
FBE has low resistance to abrasive stresses and significant damage to FBE-coated pipes occurs after brief exposure to mechanical stresses [32]. With increased coating system requirements such as high temperature use, and improved resistance to abrasion and chemicals, three-layer polyolefins (3LPO) systems become the preferred choice [1]. A 3LPO coating typically consists of an FBE primer, a polyolefin adhesive, and a polyolefin topcoat—mainly high-density polyethylene (HDPE) or polypropylene (PP). Each successive layer is designed to adhere to the next by gradually altering the chemistry to aid compatibility and chemical interaction from the steel pipe surface to the outer layer topcoat [61][62]. The multilayered approach requires minimum interfacial stresses between layers to ensure the final coating can achieve optimal adhesion and barrier properties. Design parameters such as the thickness of each layer and application temperature are thus of equal importance to the layer compositions [1]. Finite element modelling of a 3LPO coating showed that increasing the polyethylene (PE) topcoat layer thickness reduces the value of stress at the PE/epoxy interface but increases the stress at the epoxy/steel interface [63]. Processing of polymer structures at temperatures high above their Tgs can result in mutual interdiffusion of two distinct polymers across an interface. For example, a diffusive interphase layer of 10–1000 Å leads to strong entanglements between two compatible polymers [64].
Another multi-component system is HPPC. It is expected to have low susceptibility to internal stress development over wide temperature variations. It typically consists of a 175–250 µm FBE primer, a 125–150 µm polyolefin powder adhesive, and a 500–800 µm topcoat HDPE, all applied by electrostatic spray on the heated pipeline [31]. The desired thickness for each layer is selected according to project specifications and performance requirements—e.g., thicker topcoat HDPE profiles at areas susceptible to mechanical damage, weathering, or high humidity. This coating system benefits from the directional solidification of the polymer, which protects the coating from internal stresses and microvoids [31]. Mass transport studies on HPPC generally focus on coating performance in CD tests [7][65]. Permeability data for this coating at higher temperatures are limited and have been measured qualitatively, mostly relying on comparison with other coating structures [26]. Thorough investigation of mass transport in multilayered coatings requires empirical data on the barrier properties of PE components.
5. Coating Imperfections and Remaining Life Assessments
The literature concerning the time-dependent barrier performance of an a priori defect-free coating system is relatively inadequate. In other words, assuming that a near-perfect coating system is placed on a well-prepared steel substrate, one is not likely to find studies addressing questions such as: How long could a pipeline operator reasonably count on the coating retaining its initial, “ideal”, barrier properties? What effect might cathodic protection have on the deterioration of these properties, among other considerations? A modelling scheme to answer such questions requires performance assessments of the coating during consecutive steps of degradation. It also needs to connect mass transfer to the degradation process and predict the frequency of coating failures. To accomplish such objectives, possible modes of failure, as well as experimental approaches to generate attributes for probability of such events, need to be identified for a coating system of interest. For example, introduction of new interfaces between coating layers through the multilayering approach may increase the probability of developing defects, i.e., porosity in forming layers and insufficient adhesion between layers in the coating profile. Although effective pipe inspections minimize the occurrence of defects for in-service pipelines, progressive degradation can induce imperfections in the coating structure. An exemplar of such cases is sequential water absorption/desorption, which can develop internal stresses in the coating profile. This eventually leads to adhesion loss or microcracks in the final stage of degradation [66][67]. UV exposure also causes loss of mechanical properties and thereby lowers the durability of the coating [68]. Complementary mechanical properties tests such as adhesion pull-off and nanoindentation techniques can quantitatively show this decline [69][70]. In nanoindentation tests, for instance, the resistance of the coating layer against indentation force can be used as a proxy for the extent of degradation of its mechanical properties.
Predicting the remaining life of pipelines using numerical assessments has received attention in recent research [71][72][73]. The modelling scheme to predict the failure point can follow either deterministic or probabilistic approaches [74][75]. However, it is generally acknowledged that assessments for an existing structure referencing back to design specifications (as in deterministic methods) for such complicated systems are likely to result in excessively conservative estimates of remaining life [76]; they are likely to require more structural capacity than is reasonably necessary to fulfill both safety and performance criteria. Common modes of failure for each coating type have been documented in some existing reports [77][78].
This review addresses the consequences of exposing a coating system to challenging environments, however, diverse mechanisms of coating degradation indicate that one cannot directly use the transport data, if known, to formulate a model for the overall degradation process. Alternatively, probabilistic methods to relate likelihood of failures based on the declining properties of the coating appear to be a good fit [79][80]. Monte Carlo simulation is a widely used technique for risk assessment and reliability analyses in disparate engineering fields and is successfully used for lifetime predictions in oil and gas pipelines (i.e., to process corrosion wastage after coating failure) [81][82][83]. A modeling framework to address individual failure types based on mass transport analyses and mechanistic attributes of degradation (i.e., corrosion attack or disbondment) can generate signals for an ultimate failure (e.g., exposed pipe). In theory, a failure probability is determined by the variability of environmental parameters (e.g., history of humidity, temperature, UV exposure, etc.) and their interactions with mass transport properties of the coatings. Independent statistical distributions based on the empirical data generate a cumulative distribution function to predict the failure behavior of the coating [84][85]. Numerical solutions of the resulting evolution equation (due to gradual degradation) can be achieved by employing computational algorithms [86][87]. Such a numerical study is missing in coating degradation literature and would be helpful to reach optimal inspection intervals for oil and gas pipelines.
References
- Varley, R.J.; Leong, K.H. Polymer Coatings for Oilfield Pipelines. In Active Protective Coatings: New-Generation Coatings for Metals; Hughes, A.E., Mol, J.M.C., Zheludkevich, M.L., Buchheit, R.G., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 385–428. ISBN 9789401775380.
- Allcock, B.W.; Lavin, P.A. Novel composite coating technology in primary and conversion industry applications. Surf. Coat. Technol. 2003, 163, 62–66.
- Romano, M.; Goldie, B.; Kehr, A.; Roche, M.; Papavinasam, S.; Attard, M.; Balducci, B.; Revie, R.W.; Melot, D.; Paugam, G. Protecting and Maintaining Transmission Pipeline. J. Prot. Coat. Linings e-book 2012.
- Popov, B.N. Organic coatings. In Corrosion Engineering—Principles and Solved Problems; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781420094633.
- Sid Harris Powder Coatings Meet All Industrial Coating Requirements! Focus Powder Coat. 2012, 1–2.
- Husain, A.; Al-Bahar, S.; Chakkamalayath, J.; Vikraman, A.; Al Ghamdi, A.; Kamshad, T.; Siriki, R.S. Differential scanning calorimetry and optical photo microscopy examination for the analysis of failure of fusion bonded powder epoxy internal coating. Eng. Fail. Anal. 2015, 56, 375–383.
- Niu, L.; Cheng, Y.F. Development of innovative coating technology for pipeline operation crossing the permafrost terrain. Constr. Build. Mater. 2008, 22, 417–422.
- Kehr, A. Fusion-bonded epoxy internal linings and external coatings for pipeline corrosion protection. In Piping Handbook, 7th ed.; McGraw-Hill Handbooks: New York, NY, USA, 1999; pp. B481–B505.
- Zhou, W.; Edmondson, S.J.; Jeffers, T.E. Effects of Application Temperature, Degree of Cure and Film Thickness on Cathodic Disbondment of Conventional and New Generation FBE Coatings. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: San Diego, CA, USA, 2006.
- Waslen, D. External Coatings. In Oil and Gas Pipelines: Integrity and Safety Handbook; Winston, R.R., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 439–446. ISBN 978-1-118-21671-2.
- Verma, C.; Olasunkanmi, L.O.; Akpan, E.D.; Quraishi, M.A.; Dagdag, O.; El Gouri, M.; Sherif, E.S.M.; Ebenso, E.E. Epoxy resins as anticorrosive polymeric materials: A review. React. Funct. Polym. 2020, 156, 104741.
- Armelin, E.; Alemán, C.; Iribarren, J.I. Anticorrosion performances of epoxy coatings modified with polyaniline: A comparison between the emeraldine base and salt forms. Prog. Org. Coat. 2009, 65, 88–93.
- Aguirre-Vargas, F. Thermoset coatings. In Thermosets: Structure, Properties, and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 369–400. ISBN 9780081010211.
- Jasiukaitytė-Grojzdek, E.; Huš, M.; Grilc, M.; Likozar, B. Acid-catalysed α-O-4 aryl-ether bond cleavage in methanol/(aqueous) ethanol: Understanding depolymerisation of a lignin model compound during organosolv pretreatment. Sci. Rep. 2020, 10, 1–12.
- Jasiukaitytė-Grojzdek, E.; Huš, M.; Grilc, M.; Likozar, B. Acid-Catalyzed α-O-4 Aryl-Ether Cleavage Mechanisms in (Aqueous) γ-Valerolactone: Catalytic Depolymerization Reactions of Lignin Model Compound during Organosolv Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 17475–17486.
- Bjelić, A.; Hočevar, B.; Grilc, M.; Novak, U.; Likozar, B. A review of sustainable lignocellulose biorefining applying (natural) deep eutectic solvents (DESs) for separations, catalysis and enzymatic biotransformation processes. Rev. Chem. Eng. 2020.
- Saliba, P.A.; Mansur, A.A.; Santos, D.B.; Mansur, H.S. Fusion-bonded epoxy composite coatings on chemically functionalized API steel surfaces for potential deep-water petroleum exploration. Appl. Adhes. Sci. 2015, 3.
- Saliba, P.A.; Mansur, A.A.P.; Mansur, H.S. Advanced Nanocomposite Coatings of Fusion Bonded Epoxy Reinforced with Amino-Functionalized Nanoparticles for Applications in Underwater Oil Pipelines. J. Nanomater. 2016, 2016.
- Kehr, J.A. Fusion-Bonded Epoxy (FBE): A Foundation for Pipeline Corrosion Protection; NACE International: Houston, TX, USA, 2003; ISBN 157590148X.
- Ubaid, F.; Radwan, A.B.; Naeem, N.; Shakoor, R.A.; Ahmad, Z.; Montemor, M.F.; Kahraman, R.; Abdullah, A.M.; Soliman, A. Multifunctional self-healing polymeric nanocomposite coatings for corrosion inhibition of steel. Surf. Coat. Technol. 2019, 372, 121–133.
- Schultze, J.D.; Böhning, M.; Springer, J. Sorption and permeation properties of poly(p-phenylene sulfide) crystallized in the presence of sorbed gas molecules. Die Makromol. Chem. 1993, 194, 431–444.
- Naito, Y.; Kamiya, Y.; Terada, K.; Mizoguchi, K.; Wang, J. Pressure dependence of gas permeability in a rubbery polymer. J. Appl. Polym. Sci. 1996, 61, 945–950.
- Sangaj, N.S.; Malshe, V.C. Permeability of polymers in protective organic coatings. Prog. Org. Coat. 2004, 50, 28–39.
- Husain, A.; Chakkamalayath, J.; Al-Bahar, S. Electrochemical impedance spectroscopy as a rapid technique for evaluating the failure of fusion bonded epoxy powder coating. Eng. Fail. Anal. 2017, 82, 765–775.
- Jadoon, A.N.K.; Thompson, I. Fusion bonded epoxy mainline and field joint coatings performance from the X100 field trial—A case study. Int. J. Press. Vessel. Pip. 2012, 92, 48–55.
- Fu, A.Q.; Cheng, Y.F. Characterization of the permeability of a high performance composite coating to cathodic protection and its implications on pipeline integrity. Prog. Org. Coat. 2011, 72, 423–428.
- Legghe, E.; Aragon, E.; Bélec, L.; Margaillan, A.; Melot, D. Correlation between water diffusion and adhesion loss: Study of an epoxy primer on steel. Prog. Org. Coat. 2009, 66, 276–280.
- Varughese, K. Improving functional powder coatings’ corrosion protection. Prod. Finish. 2000, 64, 49–54.
- Guan, S.; Mayes, P.; Andrenacci, A.; Wong, D.; Shawcor, B.S. Advanced Two Layer Polyethylene Coating Technology for Pipeline Protection. In Proceedings of the International Corrosion Control Conference, Sydney, Australia, 25–28 November 2007; pp. 1–7.
- Guan, S.W.; Kehr, A.J. High temperature cathodic disbondment testing for pipeline coatings. In Proceedings of the Corrosion Conference, San Antonio, TX, USA, 1–17 March 2014.
- Singh, P.; Haberer, S.; Gritis, N.; Worthingham, R.; Cetiner, M. New developments in high performance coatings. In Proceedings of the BHR Group 2005, Pipeline Protect, Paphos, Cyprus, 2–4 November 2005; Volume 16, pp. 55–66.
- Andrenacci, A.; Wong, D.T. Simulation of coating behavior in buried service environment. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: Nashville, TN, USA, 2007; pp. 1–14.
- Lam, C.N.C.; Wong, D.T.; Steele, R.E.; Edmondson, S.J. A New Approach To High Performance Polyolefin Coatings. In Proceedings of the Corrosion Conference and Expo; NACE International; NACE International: Nashville, TN, USA, 2007.
- Batallas, M.; Singh, P. Evaluation of Anticorrosion Coatings For High Temperature Service. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: New Orleans, LA, USA, 2008; pp. 1–16.
- Linde, E.; Giron, N.H.; Celina, M.C. Water diffusion with temperature enabling predictions for sorption and transport behavior in thermoset materials. Polymer 2018, 153, 653–667.
- Carfagna, C.; Apicella, A. Physical Degradation by Water Clustering in Epoxy Resins. J. Appl. Polym. Sci. 1983, 28, 2881–2885.
- Perdomo, J.J.; Song, I. Chemical and electrochemical conditions on steel under disbonded coatings: The effect of applied potential, solution resistivity, crevice thickness and holiday size. Corros. Sci. 2000, 42, 1389–1415.
- Asrar, N.; MacKay, B.; Øystein, B.; Stipaničev, M.; Jackson, J.E.; Jenkins, A.; Scheie, J.; Vittonato, J. Corrosion—The Longest War. Oilf. Rev. 2016, 28, 34–49.
- Malshe, V.C.; Sangaj, N.S. A simple method for detection of the onset of corrosion of mild steel panel. Prog. Org. Coat. 2005, 53, 312–314.
- Pauly, S. Permeability and diffusion data. In Polymer Handbook; Brandrup, J., Immergut, E.H., Eds.; Wiley: New York, NY, USA, 1999; pp. 543–569. ISBN 9788578110796.
- Thomas, N.L. The barrier properties of paint coatings. Prog. Org. Coat. 1991, 19, 101–121.
- Aksu, E. Thermosets for pipeline corrosion protection. In Thermosets; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 453–476. ISBN 978-0-08-101021-1.
- Kehr, J.A.; Enos, D.G. FBE, a foundation for pipeline corrosion coating. In Proceedings of the Corrosion, Orlando, FL, USA, 26–31 March 2000; pp. 1–20.
- Wong, D.; Lam, C.; Singh, P. Development of Low Application Temperature Coating Systems for Steel Pipelines. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: Phoenix, AZ, USA, 2018; pp. 1–10.
- Zhou, W.; Jeffers, T.E.; Decker, O.H. Properties of a Novel High Tg FBE Coating for High Temperature Service. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: Nashville, TN, USA, 2007; pp. 1–8.
- Wei, Y.H.; Zhang, L.X.; Ke, W. Evaluation of corrosion protection of carbon black filled fusion-bonded epoxy coatings on mild steel during exposure to a quiescent 3% NaCl solution. Corros. Sci. 2007, 49, 287–302.
- Wang, D.; Sikora, E.; Shaw, B. A study of the effects of filler particles on the degradation mechanisms of powder epoxy novolac coating systems under corrosion and erosion. Prog. Org. Coat. 2018, 121, 97–104.
- Luo, S.; Zheng, Y.; Li, J.; Ke, W. Slurry erosion resistance of fusion-bonded epoxy powder coating. Wear 2001, 249, 733–738.
- Luo, S.; Zheng, Y.; Li, J.; Ke, W. Effect of curing degree and fillers on slurry erosion behavior of fusion-bonded epoxy powder coatings. Wear 2003, 254, 292–297.
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–24.
- Yan, M.; Sun, C.; Xu, J.; Wu, T.; Yang, S.; Ke, W. Stress corrosion of pipeline steel under occluded coating disbondment in a red soil environment. Corros. Sci. 2015, 93, 27–38.
- Kanehashi, S.; Nagai, K. Analysis of dual-mode model parameters for gas sorption in glassy polymers. J. Memb. Sci. 2005, 253, 117–138.
- Tsujita, Y. Gas sorption and permeation of glassy polymers with microvoids. Prog. Polym. Sci. 2003, 28, 1377–1401.
- Cetiner, M.; Singh, P.; Abes, J.; Gilroy-Scott, A. UV Degradation of Fusion Bonded Epoxy Coating in Stockpiled Pipes. In Proceedings of the 2000 3rd International Pipeline Conference; ASME: Calgary, AB, Canada, 2000; Volume 2, pp. 691–702.
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761.
- Rowe, B.W.; Freeman, B.D.; Paul, D.R. Physical aging of ultrathin glassy polymer films tracked by gas permeability. Polymer 2009, 50, 5565–5575.
- Latino, M.; Varela, F.; Tan, Y.; Forsyth, M. The effect of ageing on cathodic protection shielding by fusion bonded epoxy coatings. Prog. Org. Coat. 2019, 134, 58–65.
- Damian, C.; Espuche, E.; Escoubes, M. Influence of three ageing types (thermal oxidation, radiochemical and hydrolytic ageing) on the structure and gas transport properties of epoxy–amine networks. Polym. Degrad. Stab. 2001, 72, 447–458.
- Kirkpatrick, D.; Aguirre, F.; Jacob, G. Review of epoxy polymer thermal aging behavior relevant to fusion bonded epoxy coatings. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: New Orleans, LA, USA, 2008; pp. 1–12.
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part II: Variations of glass transition temeprature. Polymer 1999, 40, 5505–5512.
- Guan, S.W.; Bacon, T.; Chen, K.Y.; Mclennan, S.; Uppal, N. Preservation of Coated Pipes for Long Term Storage in Tropical Environment. In Proceedings of the NACE International East Asia Pacific Rim Area, Bali, Indonesia, 2–4 September 2014.
- Guan, S.W.; Kehr, A.J. High-Temperature Cathodic Disbondment Testing—Part 2: Parallel Testing. Mater. Perform. 2015, 54, 2–6.
- Legghe, E.; Joliff, Y.; Belec, L.; Aragon, E. Computational analysis of a three-layer pipeline coating: Internal stresses generated during the manufacturing process. Comput. Mater. Sci. 2011, 50, 1533–1542.
- Deng, S.; Djukic, L.; Paton, R.; Ye, L. Thermoplastic–epoxy interactions and their potential applications in joining composite structures—A review. Compos. Part A 2015, 68, 121–132.
- Latino, M.; Varela, F.; Forsyth, M.; Tan, Y. Self-validating electrochemical methodology for quantifying ionic currents through pipeline coatings. Prog. Org. Coat. 2018, 120, 153–159.
- Diodjo, M.R.T.; Joliff, Y.; Belec, L.; Aragon, E.; Perrin, F.X.; Bonnaudet, M.; Lanarde, L. Numerical modeling of stresses relaxation phenomena in the complex assembly steel pipe/three layers polyethylene coating. Prog. Org. Coat. 2017, 104, 152–160.
- Joliff, Y.; Belec, L.; Aragon, E. Influence of the thickness of pipeline coating on internal stresses during the manufacturing process by finite element analysis. Comput. Mater. Sci. 2013, 68, 342–349.
- Guermazi, N.; Elleuch, K.; Ayedi, H.F. The effect of time and aging temperature on structural and mechanical properties of pipeline coating. Mater. Des. 2009, 30, 2006–2010.
- Abdou, M.I.; Ayad, M.I.; Diab, A.S.M.; Hassan, I.A.; Fadl, A.M. Influence of surface modified ilmenite/melamine formaldehyde composite on the anti-corrosion and mechanical properties of conventional polyamine cured epoxy for internal coating of gas and oil transmission pipelines. Prog. Org. Coat. 2017, 113, 1–14.
- Samad, U.A.; Alam, M.A.; Chafidz, A.; Al-Zahrani, S.M.; Alharthi, N.H. Enhancing mechanical properties of epoxy/polyaniline coating with addition of ZnO nanoparticles: Nanoindentation characterization. Prog. Org. Coat. 2018, 119, 109–115.
- Liu, A.; Chen, K.; Huang, X.; Chen, J.; Zhou, J.; Xu, W. Corrosion failure probability analysis of buried gas pipelines based on subset simulation. J. Loss Prev. Process Ind. 2019, 57, 25–33.
- Zeng, Y.; Zhang, D.W.; Dai, J.G.; Fang, M.S.; Jin, W.L. Determining the service life extension of silane treated concrete structures: A probabilistic approach. Constr. Build. Mater. 2020, 249, 118802.
- Lampe, J.; Hamann, R. Probabilistic model for corrosion degradation of tanker and bulk carrier. Mar. Struct. 2018, 61, 309–325.
- Wang, Y.; Zhang, P.; Qin, G. Non-probabilistic time-dependent reliability analysis for suspended pipeline with corrosion defects based on interval model. Process Saf. Environ. Prot. 2019, 124, 290–298.
- Hu, J.; Tian, Y.; Teng, H.; Yu, L.; Zheng, M. The probabilistic life time prediction model of oil pipeline due to local corrosion crack. Theor. Appl. Fract. Mech. 2014, 70, 10–18.
- Melchers, R.E.; Jeffrey, R.J. Probabilistic models for steel corrosion loss and pitting of marine infrastructure. Reliab. Eng. Syst. Saf. 2008, 93, 423–432.
- Zamanzadeh, M.; Xu, H. Fusion Bonded Epoxy Coatings (FBE) and Disbondment. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: Vancouver, BC, Canada, 2016; pp. 1–11.
- Aguirre, F.; Kirkpatrick, D. Accelerated Aging of Fusion Bonded Epoxy Coating. In Proceedings of the NACE—International Corrosion Conference & Expo; NACE International: Houston, TX, USA, 2011; pp. 1–8.
- Li, L.; Yu, Y.; Wu, Q.; Zhan, G.; Li, S. Effect of chemical structure on the water sorption of amine-cured epoxy resins. Corros. Sci. 2009, 51, 3000–3006.
- Milenin, A.; Velikoivanenko, E.; Rozynka, G.; Pivtorak, N. Probabilistic procedure for numerical assessment of corroded pipeline strength and operability. Int. J. Press. Vessel. Pip. 2019, 171, 60–68.
- Syed, Z.; Lawryshyn, Y. Risk analysis of an underground gas storage facility using a physics-based system performance model and Monte Carlo simulation. Reliab. Eng. Syst. Saf. 2020, 199, 106792.
- Tee, K.F.; Khan, L.R.; Li, H. Application of subset simulation in reliability estimation of underground pipelines. Reliab. Eng. Syst. Saf. 2014, 130, 125–131.
- Li, Y.; Zhang, Y.; Kennedy, D. Reliability analysis of subsea pipelines under spatially varying ground motions by using subset simulation. Reliab. Eng. Syst. Saf. 2018, 172, 74–83.
- Seidenberger, K.; Wilhelm, F.; Schmitt, T.; Lehnert, W.; Scholta, J. Estimation of water distribution and degradation mechanisms in polymer electrolyte membrane fuel cell gas diffusion layers using a 3D Monte Carlo model. J. Power Sources 2011, 196, 5317–5324.
- Zennaro, F.M.; Ivanovska, M.; Jøsang, A. An empirical evaluation of the approximation of subjective logic operators using Monte Carlo simulations. Int. J. Approx. Reason. 2019, 111, 56–77.
- Vinu, R.; Levine, S.E.; Wang, L.; Broadbelt, L.J. Detailed mechanistic modeling of poly(styrene peroxide) pyrolysis using kinetic Monte Carlo simulation. Chem. Eng. Sci. 2012, 69, 456–471.
- Makki, H.; Adema, K.N.S.; Peters, E.A.J.F.; Laven, J.; Van Der Ven, L.G.J.; Van Benthem, R.A.T.M.; De With, G. A simulation approach to study photo-degradation processes of polymeric coatings. Polym. Degrad. Stab. 2014, 105, 68–79.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
27 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




