Retinopathy of prematurity (ROP) is an ocular vascular disease affecting premature infants, characterized by pathological retinal neovascularization (RNV), dilated and tortuous retinal blood vessels, and retinal or vitreous hemorrhages that may lead to retinal detachment, vision impairment and blindness.
1. Introduction
Retinopathy of prematurity (ROP) with pathological retinal neovascularization (RNV) is the most common cause of blindness in children, primarily afflicting preterm infants
[1]. During ROP, abnormal retinal blood vessels form sprouts and branches that extend not only over the retinal surface but also into the vitreous as they progress to pathological RNV and intravitreal neovascularization (IVNV)
[2]. In contrast to other ocular neovascular diseases, including proliferative diabetic retinopathy (DR) with RNV and wet age-related macular degeneration (AMD) with choroidal neovascularization (CNV), ROP in the developing retina of premature infants is unique because of the coexistence of physiological and pathological angiogenesis. The former is essential for normal retinal development.
ROP first appeared as a clinical disorder in the 1940s, coincident with the liberal use of supplemental oxygen to improve the survival of preterm infants with respiratory distress syndrome (RDS)
[3]. The disease typically occurs in preterm subjects that weigh about 1250 g or less and are born before 31 weeks of gestational age (GA). A full-term human pregnancy has a gestation period of ~40 weeks. Birth weight correlates inversely with the probability of ROP. There were ~14.9 million live premature births globally in 2010
[4], of which an estimated 184,700 developed ROP, including 20,000 with severe visual impairment and/or blindness
[5]. Although about 90% of ROP infants sustain only mild visual impairment, subjects with untreated RNV remain at an elevated risk for progression to more severe conditions, including dilated and tortuous retinal vessels (i.e., the “plus” disease), retinal folds, pathological RNV and IVNV, and retinal or vitreous hemorrhage that may lead to retinal detachment, impaired vision and blindness ().
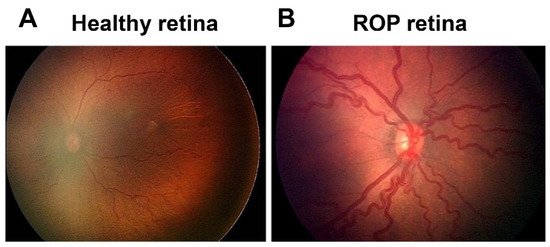
Figure 1. Fundus image of retinopathy of prematurity (ROP): (A) Healthy retina and (B) ROP retina with tortuous arteries and dilated veins as plus disease.
ROP is currently treated by laser therapy or cryotherapy that provide only limited efficacy and significant risk of adverse side effects. Anti-vascular endothelial growth factor (VEGF) drugs have been used off-label in the U.S. but are not FDA approved because of the potential for adverse side effects caused by indiscriminate inhibition of all forms of angiogenesis in the developing retina. The recent discovery by our group that secretogranin III (Scg3) is a disease-selective angiogenic factor presents a unique opportunity to selectively target and alleviate ROP safely without affecting physiological angiogenesis and normal retinal development.
2. Molecular Mechanisms of ROP
2.1. Oxygen
After low GA and birth weight, oxygen is the single most important risk factor for ROP. The liberal use of supplement oxygen in the 1940s resulted in a high incidence of ROP, first manifested as retrolental fibroplasia
[3]. The subsequent development of technologies to better monitor and regulate oxygen exposure to premature infants decreased the risk of ROP, but reduction of supplemental oxygen also increased preterm infant mortality rates
[6][7]. Clinical findings show that fluctuations of blood oxygen saturation (SpO
2) of preterm babies during the first few weeks of life also contribute to the risk of ROP, and a high incidence of intermittent hypoxia is associated with severe ROP
[8][9].
2.2. VEGF
VEGF is the most intensely studied angiogenic factor and widely considered to be the central player in both physiological and pathological angiogenesis of ROP as well as numerous other neovascular disorders. It was initially discovered as a vascular permeability factor and subsequently characterized as a potent mitogenic factor that stimulates endothelial proliferation and angiogenesis
[10]. The mammalian VEGF family comprises five members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF). Human VEGF-A undergoes alternative exon splicing into multiple variants, including VEGF111, VEGF121, VEGF145, VEGF165 and VEGF189
[10]. Of these isoforms, VEGF165 is the most active in terms of receptor stimulation and bioavailability. VEGF binds to 3 receptor tyrosine kinases, including VEGF receptor 1–3 (VEGFR1–3) encoded by
Flt1,
Kdr and
Flt4 genes, respectively
[10]. VEGFR2 is the principal conduit that promotes endothelial proliferation and vascular permeability.
VEGF levels are significantly elevated in the vitreous of ROP infants
[11][12]. Similarly, in mice with oxygen-induced retinopathy (OIR), a surrogate animal model of ROP, VEGF expression is suppressed by hyperoxia in Phase 1 but markedly upregulated by the conditions of relative hypoxia imposed in Phase 2
[13][14].
Oxygen tension regulates the expression of VEGF and multiple other angiogenic factors via hypoxia-inducible transcription factors (HIFs)
[15]. HIF-1, the main regulator of responses to hypoxia consists of two subunits, HIF-1α and HIF-1β, both of which are required to activate target genes and are expressed constitutively. Oxygen tensions in the normal range (normoxia) or hyperoxia, drive prolyl hydroxylation of HIF-1α via prolyl hydroxylase domain proteins. Hydroxylated HIF-1α is sequestered to the proteasome for ubiquitination and degradation such that HIF-1 regulation is inactivated by normal (aerobic) or elevated oxygen pressure
[15]. Under hypoxia, HIF-1α is stabilized and translocated to the nucleus where it heterodimerizes with HIF-1β, and the complex binds to hypoxia-responsive elements (HREs) of target genes, such as VEGF, to activate transcription. HIF-1 is induced during physiological angiogenesis as well as in Phase 2 of ROP but suppressed by supplemental oxygen during Phase 1
[16][17]. HIFs are central to both physiological and pathological angiogenesis in many organs
[18].
2.3. IGF-1
IGF-1 is a non-oxygen regulated factor that has been extensively studied in ROP
[19]. Clinical studies indicated that serum levels of IGF-1 in premature babies correlate inversely with the severity of clinical ROP
[20]. Administration of recombinant human IGF-1 reduces the risk of OIR in mice
[21]. Absence of IGF-1 in IGF-1
−/− mice delays normal retinal vascular development
[20]. IGF-1 is a permissive factor for VEGF-dependent vascular endothelial cell growth
[20][22][23].
IGF-1 binds the IGF-1 receptor (IGF-1R), and elimination of IGF-1R reduced RNV in OIR mice
[24]. Insulin-like growth factor-binding proteins 1–6 (IGFBP1–6) bind and sequester >99% of available IGF-1, thereby regulating bioavailability. IGFBP-3, the most abundant IGFBP, sequesters 75–90% of IGF-1 protein
[25][26]. IGFBP-3 concentrations are decreased in infants with ROP
[27]. Deletion of the IGFBP-3 gene in mice resulted in a dose-dependent increase of OIR-related vessel loss, while administration of IGFBP-3 promoted vessel regrowth in wild-type mice and reduced vaso-obliteration in OIR mice
[26][27].
2.4. Other Molecular Regulators
Erythropoietin, a key hematopoietic cytokine that regulates the formation of red blood cells during hematopoiesis, is also a pleiotropic growth factor that confers growth stimulation and cytoprotection to numerous cells, including endothelial cells. Chen et al. reported that EPO levels were suppressed in Phase 1 of OIR mice and markedly elevated in Phase 2
[28]. Exogenous EPO protected OIR retina in Phase 1 but worsened pathological RNV in Phase 2. EPO siRNA effectively suppressed pathological RNV in OIR mice
[29]. Recombinant human EPO (rhEPO) used to treat anemia of prematurity is a significant independent risk factor for developing ROP
[30].
ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) are essential fatty acids required for optimal health and cannot be synthesized by the body. They are structural components of the plasma membrane and critical for optimal fluidity of photoreceptor membranes, retinal integrity and visual function
[31]. ω-3 PUFAs include alpha-linolenic acid (ALA) and docosahexaenoic acid (DHA), while arachidonic acid (AA) is a ω-6 PUFA. DHA accounts for ~20% of total lipid in photoreceptor cells
[32] and can be metabolized to neuroprotectin D1, resolvin D1 and resolvin E1, potent lipid-derived cytokines that confer protection against OIR-induced RNV
[33]. Blockade of the peroxisome proliferator-activated receptor gamma (PPARγ) abrogated ω-3-mediated inhibition of pathological RNV through a VEGF-independent pathway
[34], suggesting that ω-3 regulates angiogenesis through PPARγ. A clinical study revealed that low postnatal levels of serum AA are strongly associated with ROP development
[35].