
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael T. McCurdy | + 1646 word(s) | 1646 | 2021-04-26 05:32:32 |
Video Upload Options
Sepsis is a clinical syndrome resulting from a dysregulated inflammatory response to infection. Sepsis management demands early diagnosis and timely treatment that includes source control, antimicrobial therapy, and resuscitation.
1. Introduction
Sepsis is a clinical syndrome resulting from a dysregulated inflammatory response to infection. In 2017, an estimated 48.9 million cases of sepsis accounted for 11 million (19.7%) deaths worldwide [1]. In 2013, in the United States alone, sepsis accounted for $24 billion in hospital expenditures with the financial burden rising significantly over the subsequent 5 years [2][3].
Delayed identification and incorrect treatment lead to worse outcomes, increased costs, and higher mortality [2]. Managing sepsis in the contemporary era revolves around early diagnosis, administration of antimicrobials, hemodynamic support with fluids and vasopressors, and source control via procedural drainage and removal of the inciting pathogen. While these interventions have led to a decrease in hospital mortality, significant shortcomings in early recognition and treatment of the underlying cause of sepsis remain: the biomolecular triggering and subsequent inciting of an uncontrolled inflammatory response [4]. Overreliance on culture data delays identification of an infectious etiology and increases the possibility of inappropriate antimicrobial selection. The downstream effects of delayed or inappropriate antimicrobials include emerging antimicrobial resistance, medication toxicity, adverse microbiome alterations, and ineffective therapy.
While source control on the macro scale is important, the trigger of the pathological inflammatory cascade in sepsis ultimately occurs at the molecular level. The complex interaction between infectious molecules and the immune system is often overlooked in present-day management of sepsis. Innovations that prevent or attenuate this pathological interaction, as well as novel supportive therapies that provide time for patients to recover, are essential to improve outcomes in sepsis.
2. Novel Diagnostics in Sepsis
According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), sepsis is defined as life-threatening organ dysfunction caused by the dysregulated host response to infection [5][6][7]. Early identification and diagnosis are essential, as prompt and appropriate treatment can improve survival [8]. Sepsis may result from any type of infection (most commonly bacterial) that affects the body (most commonly the lungs or urinary tract). In contrast, viral sepsis is caused by a viral infection (e.g., influenza) that also carries the potential for superimposed bacterial infection. The year 2020 highlighted the devastating impact of virally-mediated sepsis, triggered by severe acute respiratory syndrome coronavirus 2. Our review focuses mainly on contemporary novel diagnostics and therapeutics in bacterial sepsis identified in the literature search covering a one-year period (November 2019–November 2020).
Limited resources are currently available to aid in early diagnosis of sepsis. Though blood cultures can occasionally identify the responsible pathogen and direct later antimicrobial therapy, their inability to yield timely results limits their role in the initial diagnostic process. Several molecular approaches have been developed in order to improve conventional culture-based identification, including PCR and matrix-assisted laser desorption ionization–time of flight (MALDI–TOF) mass spectrometry. Although MALDI–TOF may decrease the time to result to as early as two and half hours once the blood cultures become positive [9][10], a broader clinical evaluation of this approach is still missing. Recent data suggest that transcriptomic profiling by multiplexed quantitative PCR (qPCR) and metabolite detection by liquid chromatography-tandem mass spectrometry (LC-MS/MS) have potential in the clinical development of diagnostic tests capable of overcoming the limitations of single molecules to differentiate between infectious and noninfectious causes of systemic inflammation [11].
Various clinical scoring systems, such as the sequential organ failure assessment (SOFA) score, exist to assist with the diagnosis of sepsis [5][7][12]. Although SOFA is one of many such tools, may help identify patients with increased risk of death, and is utilized in the current sepsis definitions [5], it is by no means specific for infection or sepsis [3].
Some biomarkers, such as C-reactive protein (CRP) and procalcitonin (PCT), have been widely used as an acute phase reactant in critically ill patients, but their diagnostic and prognostic values for sepsis are limited [13][14][15]. In a retrospective cohort study in critically ill patients fulfilling the Sepsis-3 criteria, the diagnostic accuracies of PCT and CRP insufficiently predicted proven infection, with no difference in decrease in both markers in 28-day survivors and nonsurvivors [16]. However, the multicenter, open-label, Procalcitonin-guided Antimicrobial Therapy to Reduce Long-Term Sequelae of Infections (PROGRESS) trial in 266 patients meeting Sepsis-3 criteria demonstrated that PCT-guided therapy, as compared to standard care, yielded a significantly reduced mortality [17]. With more than 100 biomarkers already described and proposed for sepsis [18], defining which marker may be useful to optimize diagnostics and therapeutic strategies remains a challenge.
A recent comprehensive review identified 5367 studies investigating the use of biomarkers in relation to sepsis [18], with a total of 80 new individual biomarkers emerging over the past decade. Of these 80, a mean of 21 biomarkers were assessed specifically for the diagnosis of sepsis in basic research studies, clinical studies, and studies combining both approaches. We attempted to categorize the various biomarkers according to pathophysiological roles (Figure 1), although for many, identifying a single clear role was not possible. While many studies have validated a multibiomarker-based risk model that estimates mortality probability in adults with septic shock [19], we focused on clinical studies in adults over the past year that compared biomarkers in different sepsis-related pathways. Table 1 summarizes different novel biomarkers reviewed below in detail.
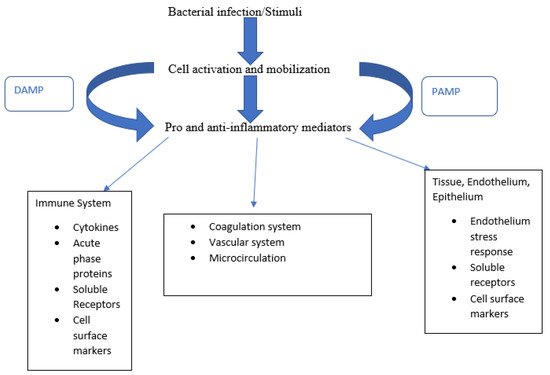
Figure 1. Biomarkers sorted according to their pathophysiological role. Bacterial stimuli cause cell activation and, along with PAMPs and DAMPs, release pro-inflammatory mediators triggering a broad host response.
Table 1. Summary of biomarkers for novel therapeutics for sepsis.
|
Summary of Biomarkers |
|
|---|---|
|
1. Innate response biomarkers |
a. Pathogen-associated molecular patterns (PAMPs) |
|
b. Damage-associated molecular patterns (DAMPs) |
|
|
c. Calprotectin |
|
|
2. Cytokine/Chemokine biomarkers |
a. Interleukin 6 (IL-6) |
|
b. Monocyte Chemoattractant Protein 1 (MCP1) |
|
|
c. Pentraxin (PTX) 3 |
|
|
d. sTNFR1 |
|
|
3. Receptor Biomarkers |
a. Presepsin |
|
b. CD64 |
|
|
c. Soluble triggering receptors expressed on myeloid cells (sTREM-1) |
|
|
d. TLR-4 |
|
|
e. PD1 |
|
|
4. Microcirculation related biomarkers |
a. Angiopoietin-1 (Ang-1) and Angiopoietin-2 (Ang-2) |
|
b. Adrenomedullin (ADM) and Pro-Adrenomedullin (ProADM) |
|
|
5. Biomarkers of Organ Dysfunction |
a. Micro-RNA (miRNA) |
|
b. Long Non-Coding RNAs (LncRNAs) |
|
|
c. Matrix Metalloproteinases (MMPs) |
|
3. Therapeutics
The mainstay of therapy in sepsis revolves around two broad principles: (1) source control to remove the infectious stimulus and (2) resuscitation optimization to both attenuate the pathologic inflammatory response and provide end-organ support. Current source control therapies include antimicrobial administration and procedural interventions to reduce the pathogenic burden [20]. Unfortunately, these therapies incompletely address the integral role of infectious molecular triggers to incite and propagate the characteristic inflammatory cascade of sepsis that manifests itself to different degrees according to each patient’s unique immune system and biochemical milieu. Meanwhile, supportive care is often limited to the implementation and titration of therapies such as intravenous fluids, vasopressors, mechanical ventilation, and renal replacement therapy (RRT). Comprehensive application of the correct combination of the above therapies in a timely manner improves outcomes in sepsis. We discuss novel therapies (Table 2) that target the pathogen burden and those that target the host by attenuating the adverse effects of the molecular triggers of inflammation to support patients until recovery.
Table 2. Summary of benefits, concerns, and current phase of clinical trials for novel therapeutics for sepsis.
|
Therapy |
Benefit |
Concern |
Phase of Clinical Trial |
|---|---|---|---|
|
PAMP Removal |
Improved hemodynamics; improved mortality in murine model |
Differing mechanisms/targets of removal between devices. No studies assessing effect on mortality to date |
Emergency Food and Drug Administration (FDA)-approval for Covid-19, ongoing multicenter clinical trials [21] |
|
Bacteriophages |
Can neutralize multidrug-resistant (MDR) bacteria |
No randomized controlled data assessing efficacy |
Case reports in humans [22] |
|
Intravenous immunoglobulin (IVIG) |
Useful in certain inflammatory conditions |
No defined benefit in sepsis patients |
FDA-approved for immunodeficiencies and inflammatory conditions |
|
Targeted Monoclonal Antibodies |
Avoids antibiotics resistance |
Each drug only effective against targeted organism |
Phase 3 trials underway [23] |
|
Liposomes |
Can bind bacterial toxin to minimize damage |
Limited use in bacteria that secrete endotoxin |
Phase 1 trials completed [24] |
|
Alkaline Phosphatase |
Mortality reduction in septic shock with acute kidney injury |
Benefit found in only those with acute kidney injury |
Phase 2 trials [25] |
|
Antimicrobial Peptides |
Synergism with antimicrobials |
Cytotoxicity towards host cells |
Phase 3 trials [26] |
|
Nanoparticles |
Increase potency and minimize side effects of antimicrobials |
High development costs |
Liposomal amphotericin B FDA-approved [27] |
|
Angiotensin II |
Catecholamine-sparing effect; improved mortality in certain patient populations |
Limited prospective experience outside of phase III trials |
FDA-approved for use in septic shock |
|
Selepressin |
Catecholamine-sparing effect with lower net fluid balance |
No change in ventilator/vasopressor-free days |
Phase 3 trial completed [28] |
|
Mesenchymal Stem Cells |
Decreased cell injury in murine sepsis models |
Concern for oncogenicity |
Phase 2 trials [23] |
|
Extracellular Vesicles |
Shown to improve renal recovery in murine models of sepsis |
No standard nomenclature/isolation techniques |
Phase 2 trials [29] |
|
TLR4 Ligand Binders |
Positive results in murine models of sepsis |
Potentially oncogenic |
FDA-approved only in the setting of cancer therapy |
|
Interleukin agonists/antagonists |
IL-7 agonist: prevents lymphopenia in septic shock; Anakinra: improved mortality in those with elevated IL-1RA levels; IL-6R and IL-6 antagonist: attenuates cytokine storm |
IL-7 agonist: No mortality benefit in current trials; Anakinra: No data for routine use in sepsis IL-6R and IL-6 antagonist: mixed data, no data for non-covid sepsis |
Phase 2 trials [30]; Anakinra FDA-approved for rheumatoid arthritis IL-6R and IL-6 antagonist: phase 2 and phase 3 trials [31]; FDA-approved for rheumatoid arthritis, EUA for Covid-19 |
|
cGAS-STING (cyclic GMP-AMP synthase-stimulator of interferon genes) |
Murine models of sepsis demonstrated survival benefit |
No in human data to suggest benefit in sepsis |
FDA-approved for non-small lung cancer |
|
Adrenomedullin |
Potential to decrease capillary permeability in sepsis |
Concern with potential of hypotension |
Phase 2 trials [23] |
|
Eculizumab |
Improved multiorgan dysfunction in Baboon models of sepsis |
May lead to immunosuppression |
FDA-approved for use in atypical hemolytic uremic syndrome |
|
Interferon Gamma |
Case series demonstrating improved cytokine profile |
No RCT studying IFN-ɣ in sepsis |
FDA-approved for chronic granulomatous disease and certain malignancies |
|
Soluble TREM-1 and Nangibotide |
Improved SOFA scores, especially in those with elevated sTREM-1 levels |
Short half-life requires infusion |
Phase 2 trials [32] |
|
Immune Checkpoint Modulators |
Improved absolute lymphocyte count (ALC) in those with low ALC and septic shock |
Patient relevant clinical outcomes unknown |
Phase 2 trials [30] |
|
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) |
Reduced length of mechanical ventilation for sepsis-induced immunosuppression |
No clear mortality benefit in sepsis |
FDA-approved for chemotherapy-induced neutropenia |
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Paoli, C.J.; Reynolds, M.A.; Sinha, M.; Gitlin, M.; Crouser, E. Epidemiology and Costs of Sepsis in the United States-An Analysis Based on Timing of Diagnosis and Severity Level. Crit. Care Med. 2018, 46, 1889–1897.
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288.
- Rhee, C.; Klompas, M. Sepsis trends: Increasing incidence and decreasing mortality, or changing denominator? J. Thorac. Dis. 2020, 12 (Suppl. 1), S89–S100.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801.
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377.
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787.
- De Backer, D.; Dorman, T. Surviving Sepsis Guidelines: A Continuous Move Toward Better Care of Patients with Sepsis. JAMA 2017, 317, 807–808.
- Burckhardt, I.; Zimmermann, S. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J. Clin. Microbiol. 2011, 49, 3321–3324.
- Faron, M.L.; Buchan, B.W.; Ledeboer, N.A. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry for Use with Positive Blood Cultures: Methodology, Performance, and Optimization. J. Clin. Microbiol. 2017, 55, 3328.
- Sutherland, A.; Thomas, M.; Brandon, R.A.; Brandon, R.B.; Lipman, J.; Tang, B.; McLean, A.; Pascoe, R.; Price, G.; Nguyen, T.; et al. Development and validation of a novel molecular biomarker diagnostic test for the early detection of sepsis. Crit. Care 2011, 15, 1–11.
- Pettilä, V. Sequential Assessment of Multiple Organ Dysfunction as a Predictor of Outcome. JAMA 2002, 287, 713–714.
- Silvestre, J.; Póvoa, P.; Coelho, L.; Almeida, E.; Moreira, P.; Fernandes, A.; Mealha, R.; Sabino, H. Is C-reactive protein a good prognostic marker in septic patients? Intensive Care Med. 2009, 35, 909–913.
- Nakamura, A.; Wada, H.; Ikejiri, M.; Hatada, T.; Sakurai, H.; Matsushima, Y.; Nishioka, J.; Maruyama, K.; Isaji, S.; Takeda, T.; et al. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock 2009, 31, 586–591.
- Henriquez-Camacho, C.; Losa, J. Biomarkers for sepsis. BioMed Res. Int. 2014, 2014, 547818.
- Van Oers, J.A.H.; de Jong, E.; Kemperman, H.; Girbes, A.R.J.; de Lange, D.W. Diagnostic Accuracy of Procalcitonin and C-reactive Protein Is Insufficient to Predict Proven Infection: A Retrospective Cohort Study in Critically Ill Patients Fulfilling the Sepsis-3 Criteria. J. Appl. Lab. Med. 2020, 5, 62–72.
- Kyriazopoulou, E.; Liaskou-Antoniou, L.; Adamis, G.; Panagaki, A.; Melachroinopoulos, N.; Drakou, E.; Marousis, K.; Chrysos, G.; Spyrou, A.; Alexiou, N.; et al. Procalcitonin to Reduce Long-Term Infection-associated Adverse Events in Sepsis. A Randomized Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 202–210.
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287.
- Wong, H.R.; Lindsell, C.J.; Pettilä, V.; Meyer, N.J.; Thair, S.A.; Karlsson, S.; Russell, J.A.; Fjell, C.D.; Boyd, J.H.; Ruokonen, E.; et al. A Multibiomarker-Based Outcome Risk Stratification Model for Adult Septic Shock. Crit. Care Med. 2014, 42, 781–789.
- Stankiewicz, J.; Jeyaraju, M.; McCurdy, M.T. SEP-1 Septic Shock Bundle Guidelines Not Applicable to Inpatients. JAMA Intern. Med. 2020, 180, 1712–1713.
- National Library of Medicine (U.S.). GARNET™ Filter (GARNET Device) IDE Used in Chronic Hemodialysis Patients with a Bloodstream Infection. Identifier NCT04658017. December 2020. Available online: (accessed on 12 January 2020).
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Sepsis, Phages, and COVID-19. Pathogens 2020, 9, 844.
- Vignon, P.; Laterre, P.F.; Daix, T.; François, B. New Agents in Development for Sepsis: Any Reason for Hope? Drugs 2020, 80, 1751–1761.
- Laterre, P.F.; Colin, G.; Dequin, P.F.; Dugernier, T.; Boulain, T.; Azeredo da Silveira, S.; Lajaunias, F.; Perez, A.; François, B. CAL02, a novel antitoxin liposomal agent, in severe pneumococcal pneumonia: A first-in-human, double-blind, placebo-controlled, randomised trial. Lancet Infect. Dis. 2019, 19, 620–630.
- Pickkers, P.; Mehta, R.L.; Murray, P.T.; Joannidis, M.; Molitoris, B.A.; Kellum, J.A.; Bachler, M.; Hoste, E.A.J.; Hoiting, O.; Krell, K.; et al. Effect of Human Recombinant Alkaline Phosphatase on 7-Day Creatinine Clearance in Patients with Sepsis-Associated Acute Kidney Injury: A Randomized Clinical Trial. JAMA 2018, 320, 1998–2009.
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047.
- Papafilippou, L.; Claxton, A.; Dark, P.; Kostarelos, K.; Hadjidemetriou, M. Nanotools for Sepsis Diagnosis and Treatment. Adv. Healthc. Mater. 2021, 10, e2001378.
- Laterre, P.F.; Berry, S.M.; Blemings, A.; Carlsen, J.E.; François, B.; Graves, T.; Jacobsen, K.; Lewis, R.J.; Opal, S.M.; Perner, A.; et al. Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients With Septic Shock: The SEPSIS-ACT Randomized Clinical Trial. JAMA 2019, 322, 1476–1485.
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21.
- Steinhagen, F.; Schmidt, S.V.; Schewe, J.C.; Peukert, K.; Klinman, D.M.; Bode, C. Immunotherapy in sepsis—Brake or accelerate? Pharmacol. Ther. 2020, 208, 107476.
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Deutschman, C.S.; et al. Cytokine elevation in severe and critical COVID-19: A rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020, 8, 1233–1244.
- François, B.; Wittebole, X.; Ferrer, R.; Mira, J.P.; Dugernier, T.; Gibot, S.; Derive, M.; Olivier, A.; Cuvier, V.; Witte, S.; et al. Nangibotide in patients with septic shock: A Phase 2a randomized controlled clinical trial. Intensive Care Med. 2020, 46, 1425–1437.




