
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Al-Shabrawey | + 2195 word(s) | 2195 | 2021-04-23 07:48:01 | | | |
| 2 | Bruce Ren | -21 word(s) | 2174 | 2021-05-07 04:48:20 | | | | |
| 3 | Conner Chen | Meta information modification | 2174 | 2021-09-22 02:29:56 | | |
Video Upload Options
Bone morphogenetic proteins (BMPs) play an important role in bone formation and repair. Recent studies underscored their essential role in the normal development of several organs and vascular homeostasis in health and diseases. Elevated levels of BMPs have been linked to the development of cardiovascular complications of diabetes mellitus. However, their particular role in the pathogenesis of microvascular dysfunction associated with diabetic retinopathy (DR) is still under-investigated.
1. Introduction
Diabetic retinopathy (DR) is one of the most common vascular complications of diabetes mellitus. The diagnosis of DR relies on the identification of microvascular abnormalities which are characterized by the breakdown of the blood retinal barrier (BRB), microaneurysms and pathological retinal neovascularization (RNV). The early stage of DR is called non-proliferative DR (NPDR), in which patients suffer from diabetic macular edema (DME) due to vascular hyperpermeability. DME is the most common cause of vision loss in diabetic patients due to the swelling and thickening of the macula that results from vascular leakage. The late stage of the disease is called proliferative DR (PDR) due to the development of RNV [1][2]. The goals of the current therapeutic strategies for DR are to prevent the inflammatory response, stabilize the BRB and prevent RNV. These goals have been achieved through laser photocoagulation and intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents, corticosteroids or both. Although anti-VEGF therapy is the current mainstay for the treatment of DR and significantly improves vision with less ocular side effects, the Diabetic Retinopathy Clinical Research Network (DRCR.net) study (Protocol I) reported that two years of anti-VEGF treatment showed ≥3-line improvement in best-corrected visual acuity (BCVA) in only ~29% of DME patients [3]. This modest response to anti-VEGF suggests that DME is multifactorial and the involvement of signaling pathways other than VEGF during the development of DR.
Intravitreal sustained release corticosteroid devices such as triamcinolone acetonide, dexamethasone and fluocinolone acetonide are beneficial, especially in patients with insufficient response to anti-VEGF, serving as anti-inflammatory agents and VEGF inhibitors [4][5]. They were reported to stabilize retinal capillaries, and hence prevent the leakage of plasma proteins into the retinal tissue [1]. Dexamethasone intravitreal implants have gained importance particularly in patients with persistent DME, although their effects were shown to be time-limited [6]. Unfortunately, increased cataract-related side effects in phakic eyes, conjunctival hemorrhage, elevated intra-ocular pressure (IOP) and ocular pain were reported [7][8][9]. Thus, uncovering other pathways that may contribute to the pathogenesis of microvascular dysfunction in DR is still of great importance and may provide novel therapeutic targets to develop new alternative treatments that may overcome the limitations of current therapies [10].
The retina is a highly specialized neuronal tissue which has the ability to convert visible light into an electrochemical signal. This signal is carried to the brain, which interprets it as vision. BRB plays a fundamental role in maintaining the privilege of the eye by the regulation of the fluid and molecular movement between ocular vascular beds and retinal tissues. The BRB includes the inner blood retinal barrier (iBRB) and the outer blood retinal barrier (oBRB). iBRB consists of tight junctions between retinal endothelial cells, pericytes and glial cells. On the other hand, retinal pigment epithelium (RPE) cells linked by tight junctions create the oBRB and rest on the underlying Bruch’s membrane. oBRB plays an essential role in maintaining the microenvironment of the outer retina, while iBRB maintains the microenvironment in the inner neural retina, and any dysfunction in iBRB or oBRB contributes to the pathophysiology of a number of retinal diseases, such as DR and AMD [11][12][13].
2. BMP Signaling Pathways
BMPs are a subgroup of the transforming growth factor β (TGFβ) superfamily. There are ~20 BMPs which share structural similarities and were first described as key players in the formation and repair of bones (Urist, 1965, Urist and Strates, 1971). BMP family members are classified according to their structural homology into numerous subgroups, including the BMP2/4 group, the BMP5/6/7/8 group, the BMP9/10 group and the BMP12/13/14 group. Different BMPs are produced as inactive large pre-pro-polypeptides (Katagiri and Watabe, 2016). The inactively synthesized BMPs contain mature polypeptides at their carboxyl terminals, with pro-domains separating them from signal peptides at their amino terminals (Xiao et al., 2007). Mature BMPs include seven cysteines, where one of them is included in the process of dimerization by a covalent disulfide bond with another BMP monomer, generating a BMP-dimer capable of binding to, and activating, a BMP receptor (Bragdon et al., 2011). BMPs have other biological functions beyond their role in bone formation and repair. For example, BMP2 is crucial for retinal development, and both BMP2 and BMP4 are implicated in the formation of cardiac septa, and their deficiency may cause heart defects in mice. Moreover, BMP7 plays a role in the development of the kidney and the heart [14][15][16]. Recently, there is a growing interest in exploring the biological functions of BMPs and their potential roles in the pathophysiology of several diseases.
BMP signaling involves a canonical pathway and a non-canonical one (Figure 1). The canonical pathway is a SMAD-dependent pathway, while the non-canonical pathway involves the activation of other non-SMAD-dependent intracellular pathways, such as MAPK, IP3/AKT [17][18][19]. This reflects the complexity of the BMP-signaling pathway and indicates the presence of possible multi-levels of regulation of this significant biological axis. BMPs are initially generated as precursor protein dimers in the cytoplasm, then cleaved to form N- and C-terminal fragments. The C-terminal fragment is the one that can bind to its receptor non-covalently. In the canonical pathway, BMPs initiate a signal transduction cascade via the formation of a hetero-tetrameric complex after binding to cell surface receptors. The complex consists of two dimers of type I and type II serine/threonine kinase receptors [20]. The mechanism of action of the heterotetrameric complex is different with different types of BMPs. Regarding BMP2 and BMP4, these molecules bind type I receptors and then recruit type II receptors to form the complex. After the formation of the receptor-complex, a type II receptor, which is constitutively active, activates a type I receptor through trans-phosphorylation. The phosphorylated type I receptor then phosphorylates downstream substrate proteins called receptor-regulated SMADs [21], including SMAD1, SMAD5 and SMAD9 (SMAD1/5/9). SMAD1/5/9 then binds to SMAD4. The nuclear translocation of the SMAD1/5/9/4 complex, which acts as a transcription factor, results in multiple downstream target genes’ expression regulation. The non-canonical pathway of BMP signaling involves the intracellular activation of the MAPK pathway, the PI3/AKT pathway, Rho-GTPases and others [18].
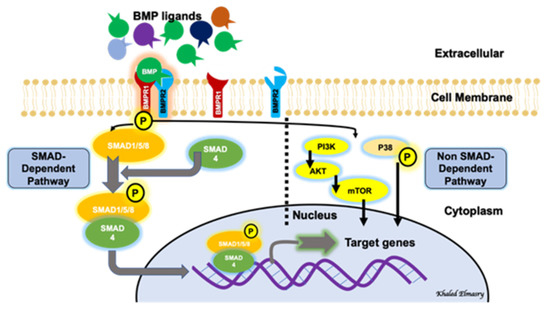
Figure 1. BMP signaling pathway involves both a canonical (SMAD-dependent) and a non-canonical (non-SMAD-dependent) pathway.
3. BMP Receptors
Studies showed that BMPs are capable of binding to two types of serine-threonine kinase BMP receptors (BMPR1 and BMPR2) [20][22]. BMPs have a higher affinity for BMPR1 than BMPR2. BMPR2 is constitutively active even in ligand absence [23]. Both receptors are structurally similar and consist of an intracellular domain with serine-threonine kinase activity, a single transmembrane domain and a short extracellular domain. BMPs can also bind to activin type II receptors ACVR2A and ACVR2B [24], which are expressed in different tissues. BMP receptors can be classified structurally into different subgroups. BMP type I receptors can be subdivided into the activin receptor-like kinase 3 (ALK3, or BMPR-IA)/ALK6 (BMPR-IB) group and the ALK1/ALK2 group. ALK2 and ALK3/6 are broadly expressed in many cell types, while ALK1 expression is mainly limited to endothelial cells [25]. BMP type II receptors (BMPR2) include BMPR-II, which is specific for BMPs, and ActR-II and ActR-IIB, which are shared by activins and myostatin [26]. Other studies identified multiple BMP co-receptors, by which BMP ligands/receptors interactions are modified. There are two co-receptors playing principal functions in vascular development and disease, including endoglin and betaglycan [27], through which BMP signaling can be activated [28][29]. Proliferating endothelial cells expressing endoglin as a transmembrane protein are capable of binding to multiple ligands, including BMP-2/7 [30].
4. BMP Signaling Regulation and Endothelial Cell Function
It was believed that the extracellular matrix (ECM) represented an inert mechanical barrier getting rid of BMPs. However, recent work demonstrated that ECM may have a role in regulating BMP signaling [31]. BMP signaling is regulated at different and multiple layers. Interestingly, the existence of inhibitory SMADs (I-SMADs), which are members of the SMAD family, plays an important regulatory function not only on BMP-signaling pathways, but also on the TGF-β superfamily regulation. I-SMADs include SMAD6 and SMAD7. Moreover, SMADs 1/5/9 are the receptor-mediated SMADs (R-SMADs), via which BMP signaling is mediated, while SMAD4 is a co-SMAD that shares in the formation of the active complex which will be translocated to the nucleus to act as a transcription factor for multiple downstream target genes. I-SMADs have conserved carboxy-terminal MH2 domains, that interact with both activated type I receptors and R-SMADs inhibiting BMP-intracellular signaling. SMAD6 inhibits the SMAD-dependent signaling pathway mediated via BMP type I receptors ALK-3 and ALK-6 [32], while SMAD7 inhibits both TGF-β- and BMP-mediated SMAD signaling pathways [33].
BMPs promote angiogenesis by facilitating endothelial motility and invasion, as well as cell proliferation [34]. During embryonic and postnatal retinal angiogenesis, a crosstalk between BMP-SMAD and Notch signaling is necessary for stalk cell specification in the endothelium [35]. Moreover, both in vitro and in vivo studies have shown that BMP2 and BMP4 mediate pro-angiogenic effects through VEGF-A/VEGFR2 and angiopoietin-1/TIE2 signaling stimulation [35][36]. BMP signal transduction is involved in the regulation of physiological as well as pathological processes of the endothelium. BMP signaling has been shown to be involved during multiple pathological conditions where vascular hyperpermeability is a typical hallmark, such as acute inflammation, atherosclerosis and metastasis [35]. However, the precise role of BMP signaling in endothelial cell permeability control remains elusive.
The interplay between BMPs and immune response should be taken into consideration. Studies on macrophages and endothelial cells have suggested both pro-inflammatory and anti-inflammatory roles [37][38]. Tumor necrosis factor (TNF)-α was found to induce the expression of BMP2 in human umbilical vein endothelial cells (HUVECs) [39] and in chondrocytes via NFκB [40], suggesting a pro-inflammatory role of BMP2.
Monocytes from type 2 diabetic patients express higher levels of BMP2. Additionally, human macrophages shift to the M1 inflammatory phenotype when exposed to high glucose. Accordingly, higher levels of BMP2 in type 2 diabetes may contribute to the activation of the inflammatory response [41][42].
Several studies have highlighted the role of BMP2 in endothelial cell inflammation [43][44][45]. Pardali et al. [39] used primary human monocytes to prove that BMP2 is a potent monocyte chemoattractant, where PI3K, P38 and MAPK are involved in signaling. They reported that BMP2 hinders macrophages’ differentiation to the M2 phenotype, which is responsible for the resolution of inflammation and healing. Moreover, they reported that BMP2 increases the adhesive properties of monocytes and endothelial cells by enhancing the expression of adhesive (ICAM-1 and VCAM-1) and pro-inflammatory (IL-1β, IL-6 and IL-8) molecules. As BMP members may exert pro-inflammatory or anti-inflammatory functions, any imbalance between these members may disturb the inflammatory response [46].
Agonists and antagonists of the TGF-β family, including BMPs, were extensively reviewed previously [47]. Among this extensive network of regulators, we would like to focus on the BMP endothelial cell precursor-derived regulator (BMPER) because it is relevant to endothelial cell function. BMPER plays an essential role in the fine-tuning of BMP activity in angiogenesis in a dose-dependent manner. BMPER was shown to have a proangiogenic effect in endothelial cells that is mediated by the activation of the fibroblast growth factor (FGF) signaling [48], and demethylation reduced BMPER expression in fibroblasts [49]. Low concentrations of BMPER promote the migration of endothelial cells, while high doses do the opposite, indicating that BMPER regulates the migration of endothelial cells in a dose-dependent manner [50]. An interesting study pointed to the role of BMPER and BMP signaling in endothelial barrier function regulation. In this study, heterozygous Bmper knockout mice were used where there was a significantly higher vascular leakage into interstitial lung tissue when compared with wild-type mice. Moreover, Bmper knockdown in endothelial cells increased endothelial permeability and reduced the VE-cadherin expression. These effects were rescued by the use of the recombinant human BMPER protein. Interestingly, enhanced BMP activity induced the effects of Bmper knockdown on both VE-cadherin expression and endothelial permeability. Increased levels of BMPER antagonized BMP4 signaling and prevented BMP4-induced endothelial barrier dysfunction and VE-cadherin downregulation, proposing BMPER as a BMP antagonist capable of restoring the endothelial barrier function [51]. Retinas from Bmper+/− mice showed increased vascular branching and sprouting. Although BMP levels did not increase in the retinas of those mice, BMP signaling was activated, as demonstrated by the increased endothelial phosphorylated SMAD protein levels and the increased expression of BMP target genes [52]. However, some studies showed that BMPER can be a BMP agonist, while other studies showed it as a BMP antagonist. An interesting study by Kelly et al. [53] postulated that BMPER switches from BMP4 activator to inhibitor when its molar concentrations exceed that of BMP4. The discrepancy among studies that examine how BMPER regulates BMP activity can be explained by a dosage-dependent molecular switch involving the BMPER-mediated internalization of BMP4. Interestingly, our recent study demonstrated a significant downregulation of the BMPER gene in cultured human retinal endothelial cells that were subjected to a high glucose treatment [54]. Taken together, these studies underscored the important role played by BMP signaling in endothelial cell function.
References
- Romero-Aroca, P.; Baget-Bernaldiz, M.; Pareja-Rios, A.; Lopez-Galvez, M.; Navarro-Gil, R.; Verges, R. Diabetic Macular Edema Pathophysiology: Vasogenic versus Inflammatory. J. Diabetes Res. 2016, 2016, 1–17.
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816.
- Gonzalez, V.H.; Campbell, J.; Holecamp, N.M.; Kiss, S.; Leowenstein, A.; Augustin, A.J.; Ma, J.; Ho, A.C.; Patel, V.; Whitcup, S.M.; et al. Early and Long-Term Responses to Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Ede-ma: Analysis of Protocol I Data. Am. J. Ophthalmol. 2016, 172, 72–79.
- Brooks, H.L.; Caballero, S.; Newell, C.K.; Steinmetz, R.L.; Watson, D.; Segal, M.S.; Harrison, J.; Scott, E.W.; Grant, M.B. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic reti-nopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch. Ophthalmol. 2004, 122, 1801–1807.
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687.
- Haller, J.A.; Kuppermann, B.D.; Blumenkranz, M.S.; Williams, G.A.; Weinberg, D.V.; Chou, C.; Whitcup, S.M. Randomized Controlled Trial of an Intravitreous Dexamethasone Drug Delivery System in Patients With Diabetic Macular Edema. Arch. Ophthalmol. 2010, 128, 289–296.
- Smithen, L.M.; Ober, M.D.; Maranan, L.; Spaide, R.F. Intravitreal triamcinolone acetonide and intraocular pressure. Am. J. Ophthalmol. 2004, 138, 740–743.
- Callanan, D.G.; Gupta, S.; Boyer, D.S.; Ciulla, T.A.; Singer, M.A.; Kuppermann, B.D.; Ching-Chi, L.; Xiao-Yan, L.; Hollander, D.A.; Schiffman, R.M.; et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse dia-betic macular edema. Ophthalmology 2013, 120, 1843–1851.
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.-Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914.
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625.
- Runkle, E.A.; Antonetti, D.A. The blood-retinal barrier: Structure and functional significance. Blood Brain Neural Barriers 2011, 686, 133–148.
- Liu, L.; Liu, X. Roles of Drug Transporters in Blood-Retinal Barrier. Adv. Exp. Med. Biol. 2019, 1141, 467–504.
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211.
- DeRubertis, F.R.; Craven, P.A.; Melham, M.F.; Salah, E.M. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: Evidence for reduced super-oxide-nitric oxide interaction. Diabetes 2004, 53, 762–768.
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes. Dis. 2014, 1, 87–105.
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect Biol. 2016, 8, a021899.
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-beta/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005.
- Zhang, Y.E. Non-Smad Signaling Pathways of the TGF-beta Family. Cold Spring Harb Perspect Biol. 2017, 9, a022129.
- Perera, N.; Ritchie, R.H.; Tate, M. The Role of Bone Morphogenetic Proteins in Diabetic Complications. ACS Pharmacol. Transl. Sci. 2020, 3, 11–20.
- Heldin, C.H.; Miyazono, K. Ten Dijke, P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997, 390, 465–471.
- Horbelt, D.; Denkis, A.; Knaus, P. A portrait of Transforming Growth Factor beta superfamily signalling: Background matters. Int. J. Biochem. Cell Biol. 2012, 44, 469–474.
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584.
- Rosenzweig, B.L.; Imamura, T.; Okadome, T.; Cox, G.N.; Yamashita, H.; Dijke, P.T.; Heldin, C.H.; Miyazono, K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 7632–7636.
- Moustakas, A. Heldin, C.H. The regulation of TGFbeta signal transduction. Development 2009, 136, 3699–3714.
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2009, 147, 35–51.
- Yu, P.B.; Beppu, H.; Kawai, N.; Li, E.; Bloch, K.D. Bone Morphogenetic Protein (BMP) Type II Receptor Deletion Reveals BMP Ligand-specific Gain of Signaling in Pulmonary Artery Smooth Muscle Cells. J. Biol. Chem. 2005, 280, 24443–24450.
- Dijke, P.T.; Goumans, M.-J.; Pardali, E. Endoglin in angiogenesis and vascular diseases. Angiogenesis 2008, 11, 79–89.
- David, L.; Mallet, C.; Mazerbourg, S.; Feige, J.J.; Bailly, S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endo-thelial cells. Blood 2007, 109, 1953–1961.
- Kirkbride, K.C.; Townsend, T.A.; Bruinsma, M.W.; Barnett, J.V.; Blobe, G.C. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008, 283, 7628–7637.
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of mul-tiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999, 274, 584–594.
- Sedlmeier, G.; Sleeman, J.P. Extracellular regulation of BMP signaling: Welcome to the matrix. Biochem. Soc. Trans. 2017, 45, 173–181.
- Goto, K.; Kamiya, Y.; Imamura, T.; Miyazono, K.; Miyazawa, K. Selective Inhibitory Effects of Smad6 on Bone Morphogenetic Protein Type I Receptors. J. Biol. Chem. 2007, 282, 20603–20611.
- Hanyu, A.; Ishidou, Y.; Ebisawa, T.; Shimanuki, T.; Imamura, T.; Miyazono, K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J. Cell Biol. 2001, 155, 1017–1027.
- Dyer, L.A.; Pi, X.; Patterson, C. The role of BMPs in endothelial cell function and dysfunction. Trends Endocrinol. Metab. 2014, 25, 472–480.
- Benn, A.; Hiepen, C.; Osterland, M.; Schütte, C.; Zwijsen, A.; Knaus, P. Role of bone morphogenetic proteins in sprouting angiogenesis: Differential BMP receptor-dependent signaling path-ways balance stalk vs. tip cell competence. FASEB J. 2017, 31, 4720–4733.
- De Vinuesa, A.G.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev. 2016, 27, 65–79.
- Li, Z.; Wang, J.; Wang, Y.; Jiang, H.; Xu, X.; Zhang, C.; Li, D.; Xu, C.; Zhang, K.; Qi, Y.; et al. Bone morphogenetic protein 4 inhibits liposaccharide-induced inflammation in the airway. Eur. J. Immunol. 2014, 44, 3283–3294.
- Helbing, T.; Arnold, L.; Wiltgen, G.; Hirschbihl, E.; Gabelmann, V.; Hornstein, A.; Esser, J.S.; Diehl, P.; Grundmann, S.; Busch, H.-J.; et al. Endothelial BMP4 Regulates Leukocyte Diapedesis and Promotes Inflammation. Inflammation. 2017, 40, 1862–1874.
- Pardali, E.; Makowski, L.M.; Leffers, M.; Borgscheiper, A.; Waltenberger, J. BMP-2 induces human mononuclear cell chemotaxis and adhesion and modulates monocyte-to-macrophage differentia-tion. J. Cell Mol. Med. 2018, 22, 5429–5438.
- Feng, J.Q.; Xing, L.; Zhang, J.-H.; Zhao, M.; Horn, D.; Chan, J.; Boyce, B.F.; Harris, S.E.; Mundy, G.R.; Chen, D. NF-kappaB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J. Biol. Chem. 2003, 278, 29130–29135.
- Torres-Castro, I.; Arroyo-Camarena, U.D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016, 176, 81–89.
- Moganti, K.; Li, F.; Schmuttermaier, C.; Riemann, S.; Klueter, H.; Gratchev, A.; Harmsen, M.C.; Kzhyshkowska, J. Hyperglycemia induces mixed M1/M2 cytokine profile in primary human monocyte-derived macrophages. Immunobiology 2017, 222, 952–959.
- Yao, Y.; Bennett, B.J.; Wang, X.; Rosenfeld, M.E.; Giachelli, C.M.; Lusis, A.J.; Boström, K.I. Inhibition of Bone Morphogenetic Proteins Protects Against Atherosclerosis and Vascular Calcification. Circ. Res. 2010, 107, 485–494.
- Boström, K.I.; Jumabay, M.; Matveyenko, A.; Nicholas, S.B.; Yao, Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ. Res. 2011, 108, 446–457.
- Derwall, M.S.; Malhotra, R.; Lai, C.S.; Beppu, Y.; Aikawa, E.; Seehra, J.S.; Zapol, W.M.; Bloch, K.D.; Yu, P.B. Inhibition of Bone Morphogenetic Protein Signaling Reduces Vascular Calcification and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 613–622.
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-beta Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140.
- Chang, C. Agonists and Antagonists of TGF-beta Family Ligands. Cold Spring Harb Perspect Biol. 2016, 8, a021923.
- Esser, J.S.; Rahner, S.; Deckler, M.; Bode, C.; Patterson, C.; Moser, M. Fibroblast Growth Factor Signaling Pathway in Endothelial Cells Is Activated by BMPER to Promote Angiogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 358–367.
- Huan, C.; Yang, T.; Liang, J.; Xie, T.; Cheng, L.; Liu, N.; Kurkciyan, A.; Mena, J.M.; Wang, C.; Dai, H.; et al. Methylation-mediated BMPER expression in fibroblast activation in vitro and lung fibrosis in mice in vivo. Sci. Rep. 2015, 5, 14910.
- Dyer, L.; Wu, Y.; Moser, M.; Patterson, C. BMPER-induced BMP signaling promotes coronary artery remodeling. Dev. Biol. 2014, 386, 385–394.
- Helbing, T.; Wiltgen, G.; Hornstein, A.; Brauers, E.Z.; Arnold, L.; Bauer, A.; Esser, J.S.; Diehl, P.; Grundmann, S.; Fink, K.; et al. Bone Morphogenetic Protein-Modulator BMPER Regulates Endothelial Barrier Function. Inflammation 2017, 40, 442–453.
- Moreno-Miralles, I.; Ren, R.; Moser, M.; Hartnett, M.E.; Patterson, C. Bone Morphogenetic Protein Endothelial Cell Precursor–Derived Regulator Regulates Retinal Angiogenesis In Vivo in a Mouse Model of Oxygen-Induced Retinopathy. Arter. Thromb. Vasc. Biol. 2011, 31, 2216–2222.
- Kelley, R.; Ren, R.; Pi, X.; Wu, Y.; Moreno, I.; Willis, M.; Moser, M.; Ross, M.; Podkowa, M.; Attisano, L.; et al. A concentration-dependent endocytic trap and sink mechanism converts Bmper from an activator to an inhibitor of Bmp signaling. J. Cell Biol. 2009, 184, 597–609.
- Al-Shabrawey, M.; Hussein, K.; Wang, F.; Wan, M.; Elmasry, K.; Elsherbiny, N.; Saleh, H.; Yu, P.B.; Tawfik, A.; Ibrahim, A.S. Bone Morphogenetic Protein-2 Induces Non-Canonical Inflammatory and Oxidative Pathways in Human Retinal Endothelial Cells. Front. Immunol. 2021, 11, 568795.




