
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barbara Costa | + 3761 word(s) | 3761 | 2021-04-21 10:28:51 | | | |
| 2 | Vivi Li | Meta information modification | 3761 | 2021-05-06 11:34:05 | | |
Video Upload Options
Neuroactive steroids are potent modulators of microglial functions and are capable of counteracting their excessive reactivity. This action has mainly been ascribed to neuroactive steroids released from other sources, as microglia have been defined unable to produce neurosteroids de novo. Unexpectedly, immortalized murine microglia recently exhibited this de novo biosynthesis; herein, de novo neurosteroidogenesis was characterized in immortalized human microglia. The results demonstrated that C20 and HMC3 microglial cells constitutively express members of the neurosteroidogenesis multiprotein machinery—in particular, the transduceosome members StAR and TSPO, and the enzyme CYP11A1. Moreover, both cell lines produce pregnenolone and transcriptionally express the enzymes involved in neurosteroidogenesis. The high TSPO expression levels observed in microglia prompted us to assess its role in de novo neurosteroidogenesis. TSPO siRNA and TSPO synthetic ligand treatments were used to reduce and prompt TSPO function, respectively. The TSPO expression downregulation compromised the de novo neurosteroidogenesis and led to an increase in StAR expression, probably as a compensatory mechanism.
1. Introduction
Microglia are specialized macrophages resident in the central nervous system (CNS). In addition to immune-related activities, microglia appear to carry out a wider spectrum of activities aimed at the physiological development and normal homeostasis of the mature CNS, including plasticity, adult neurogenesis, and cognitive processes [1][2][3]. The dysregulations of microglial activities have been proposed to promote the onset and progression of several pathological CNS conditions [2][4][5]. At steady state, microglia exhibit a resting phenotype, morphologically characterized by the presence of widely branched processes [4]. These are used by the microglia to carry out continuous surveillance of their surrounding areas. The microglia sense infection and injury and rapidly change from the resting phenotype to an activated one that is characterized by an “amoeboid” morphology [4]. This activation is a complex process characterized by the release of many pro-inflammatory mediators that contribute to the management of inflammatory processes and elimination of the pathogen (M1 phenotype) [4]. However, microglial excessive activation can cause pathological forms of neuroinflammation, contributing to the progression of neurodegenerative and tumor diseases. On the other hand, it has been proposed that the resolving phase of the pathological condition is solicited by the presence of the alternative form of activated microglia (M2 phenotype), able to release various anti-inflammatory factors, including molecules responsible for tissue repair [4][6][7]. Molecular mechanisms that regulate the transition of microglia from resting to the activated states are currently extensively debated and still poorly understood. Understanding the mechanisms underlying the modulation of these phenotypes is important in deciphering the specialized functions and exploiting their therapeutic potential [8][9]. Remarkably, what we know about microglia comes mainly from data obtained from mouse models both in vitro and in vivo, while little is known about human microglia.
The 18 kDa translocator protein (TSPO) is largely expressed in both murine and human microglia [10][11][12][13][14]; therefore, its ability to modulate microglial function and dysfunction has been considered. Notably, TSPO plays a key role in modulating inflammation, and it seems to be particularly overexpressed in neurodegenerative contexts, in which the inflammatory component stands out [11][15]. Over the years, many ligands targeting this protein have been identified and proposed for the treatment of different CNS pathologies, by virtue of their potential to improve the cellular processes promoted by TSPO functions [16][17][18]. Among these, neurosteroidogenesis seems to be the most peculiar, and the most debated one [19][20][21]. Since neurosteroidogenesis as a whole is a conserved process, its mechanism is known, albeit with some species-specific differences [22]. Neurosteroidogenesis is mediated by two mitochondrial multiprotein complexes: the transduceosome and the metabolon [20][23]. The scheme comprehensive of the currently proposed members of transduceosome and metabolon can be found in our recent review [23]. The transduceosome is responsible for the transport of free cholesterol from cytoplasm to mitochondrion surface and its transfer from the outer mitochondrial membrane (OMM) to the inner one (IMM). Even if the mechanistic details underlying the transduceosome have not been fully clarified, the StAR protein seems to move cholesterol through the OMM, while TSPO appears to regulate cholesterol translocation [23][24][25]. Following transduceosome activities, the 37 kDa StAR cytosolic precursor is processed into the 30 kDa cleaved form, a sign of successful cholesterol delivery [23][24][25]. Then, the steroidogenic metabolon, a multimeric protein complex spanning the OMM, is responsible for metabolism of cholesterol by cytochrome P450 family 11 subfamily A polypeptide 1 (CYP11A1) in the IMM. CYP11A1 is an enzyme able to convert cholesterol into pregnenolone, a reaction that is considered steroidogenesis’s rate limiting-step [20][23][25], as pregnenolone is the precursor to all neurosteroids.
Neurosteroids have been proposed as possible endogenous molecules able to contribute to the maintaining of physiological microglial activities [26][27]. Among the glial CNS component, astrocytes are the most active steroidogenic cells: in addition to the ability to synthesize pregnenolone, they can produce progesterone, dehydroepiandrosterone (DHEA), androstenedione, testosterone, and estradiol. In addition, oligodendrocytes are able to perform neurosteroidogenesis de novo, producing pregnenolone and androstenedione [22][28][29][30]. However, microglia were initially thought unable to locally produce de novo neurosteroids [31]. In fact, in basal conditions, primary cultures of murine microglia did not show gene expression for the enzyme of the rate-limiting step of steroidogenesis CYP11A1, or the enzymes involved in the metabolism of pregnenolone [31]. It was believed that microglia were able only to metabolize androgens and estrogens, using metabolites from other central and peripheral steroidogenic sources [31][32][33]. In contrast, very recent data highlighted the capacity of immortalized murine microglia to synthesize neurosteroids de novo [34]. Although little is known about the neurosteroidogenic activity of microglia yet, emerging evidence suggests that neurosteroid production could be important in maintaining CNS homeostasis and in resolving microglia-mediated inflammation [14][35].
2. Human Microglia C20 and HMC3 Cells Produce Pregnenolone De novo
As a first step, the ability of C20 and HMC3 cells to produce de novo steroids was investigated by evaluating the production of the first metabolite of neurosteroidogenesis, pregnenolone (Figure 1A,B). To quantify pregnenolone, its conversion to other metabolites of neurosteroidogenesis cascade was inhibited by the use of two synthetic compounds notoriously able to block the catalytic activity of specific steroidogenic enzymes. In particular, C20 and HMC3 were incubated in the presence of the following synthetic inhibitors: trilostane (inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD)), which prevents the conversion of pregnenolone to progesterone, and SU-1060317 (inhibitor of 17α-hydroxylase/C17–20 lyase (P450c17 or CYP17A1)), which prevents the conversion of pregnenolone into DHEA. In parallel experiments, a known human CNS neurosteroidogenic model (U87MG tumor astrocytic cell line) was used as a positive control (Figure 1C) [36].
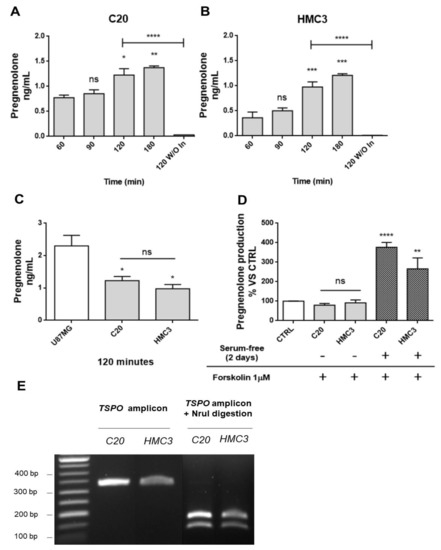
Figure 1. Human microglia C20 and HC3 cells: time-dependence of the pregnenolone production and genotyping for TSPO rs6971 polymorphism. (A,B) C20 and HMC3 cell samples were incubated in serum-free saline medium at time zero in the presence of the inhibitors trilostane and SU10603, and a kinetic analysis of pregnenolone released from microglia cells was performed. After various incubation times, the saline medium was collected from distinct cell samples and pregnenolone content was quantified by indirect ELISA. As shown in the figure, pregnenolone released from C20 and HMC3 cells increased in a time-dependent manner. A single incubation time (120 min) was conducted for the “inhibitor-free” samples named 120 without Inhibitors (120 W/O In). In this case, pregnenolone was not present in the collected samples, suggesting the conversion of pregnenolone into other neurosteroids. Pregnenolone levels were normalized based on the number of cells evaluated after crystal violet staining. Data are presented as means ± SEMs of three independent experiments. Statistical analysis was determined by one-way ANOVA followed by Bonferroni’s post-test: * p < 0.05, ** p < 0.01, *** p < 0.001 vs. 60 min; **** p < 0.0001, 120 W/O In vs. 120 min. (C) Comparison of pregnenolone production between human microglial and U87MG cells. (D) The classical stimulus of peripheral steroidogenesis (cAMP pathway activation) did not promote neurosteroidogenesis in microglial cells. Indeed, pregnenolone production by microglial cells was not stimulated following treatment with the known adenylate cyclase activator forskolin. However, the starvation phase before forskolin treatment led to a high increase in pregnenolone production. Data are represented as means ± SEMs of two independent experiments. The significance of the differences was determined by one-way ANOVA, which was followed by Bonferroni’s post-test: ** p < 0.01, **** p < 0.0001 vs. control. (E) The C20 and HMC3 cell genotyping for TSPO rs6971 was performed by restriction fragment length polymorphism (RFLP) analysis. The amplification product (329 bp) derived from genomic DNA was subjected to digestion by the restriction endonuclease NruI. Following the enzymatic digestion, the samples were subjected to agarose gel electrophoresis. Only the amplification product containing an Ala147 allele can be digested by NruI and generates restriction fragments (184 and 145 bp). As shown in the figure, C20 and HMC3 cells exhibited the restriction pattern typical of the Ala147 homozygous genotype.
First, a kinetic measurement of pregnenolone released from C20 and HMC3 cells was performed. To that end, the complete culture medium was replaced with the saline medium (time 0). Then, the conditioned saline medium was collected at different times (time 60, 90, 120, or 180 min) and subjected to pregnenolone content quantification. The results showed similar kinetics for pregnenolone production in C20 and HMC3 cells: the release of the metabolite was augmented in a time-dependent manner, reaching a statistically significant increase after 120 and 180 min (Figure 1A,B). For inhibitor-free samples, the data showed that the C20 and HMC3 cell conditioned medium collected after a single incubation time (120 min) contained very low pregnenolone levels, below or slightly above the level of detection. Overall, these findings suggested that C20 and HMC3 cells produce pregnenolone de novo and can metabolize it to generate other products of the steroidogenic cascade. Similar amounts of pregnenolone were produced by C20 and HMC3 cells after 120 min (Figure 1A,B). Such quantities were in line with those previously documented for other murine central steroidogenic primary cell models, including microglia, using a series of experimental approaches [28][34][37][38][39]. As expected, the amount of pregnenolone observed for microglial cells was lower than the amount measured in the human astrocytic tumor cell line U87MG (Figure 1C).
To investigate whether the cAMP-mediated elective transduction pathway of peripheral steroidogenic sources could stimulate microglial de novo neurosteroidogenesis, C20 and HMC3 cells were exposed to the known adenylate cyclase activator forskolin. As shown in Figure 1D, forskolin treatment did not stimulate pregnenolone production in any of the microglial cell lines [39]. However, when the same treatment was applied to microglial cells, which were previously cultured in conditions that polarize them towards an activated phenotype [40], a strong increase in pregnenolone production was observed. This suggested that only activated microglial cells acquire the functional characteristics necessary to stimulate neurosteroidogenesis through the cAMP-mediated pathway.
The known TSPO single nucleotide polymorphism rs6971 has been functionally related to de novo steroidogenic efficiency [20][41]. This polymorphism substitutes Ala with Thr at amino acid position 147 (Ala147Thr), which is localized near the cholesterol binding CRAC domain. The TSPO polymorphism Ala147Thr has been documented to affect cholesterol binding and impair the rate of steroid synthesis [20][41]. Here, genotyping analysis revealed that C20 and HMC3 cells were homozygous for the common allele coding for Ala147, suggesting that both microglial clones exhibit the genotype associated with de novo steroidogenic efficiency (Figure 1E).
3. C20 and HMC3 Cells Express Transduceosome/Metabolon Key Proteins
The expression of proteins involved in the initial phases of neurosteroidogenesis was explored in C20 and HMC3 cells. Particular attention was paid to key members of the transduceosome and metabolon [20][23]; StAR and TSPO were chosen as transduceosome members, while CYP11A1 was chosen as the representative metabolon protein.
C20 and HMC3 cells were cultured in complete medium and analyzed for basal expression of the above-reported transduceosome and metabolon members by Western blot and immunofluorescence analyses.
The Western blot results demonstrated that all the studied neurosteroidogenic members were constitutively expressed in C20 and HMC3 cells, showing differences, above all, for StAR and CYP11A1 (Figure 2A–E). Both the microglial cells highly expressed TSPO (Figure 2A), suggesting that high levels of this protein may be required to maintain essential basal activities. Comparable levels of the total StAR (comprising 37 and 30 kDa forms) (Figure 2B) were observed in both microglial cells. However, reduced levels of the cytosolic 37 kDa StAR (Figure 2C) and increased levels of the mitochondrial 30 kDa StAR (Figure 2D) were observed in HMC3 than in C20 cells. Noteworthily, the presence of the StAR mitochondrial form pointed towards successful cholesterol delivery. CYP11A1 expression was higher in HMC3 than C20 cells.
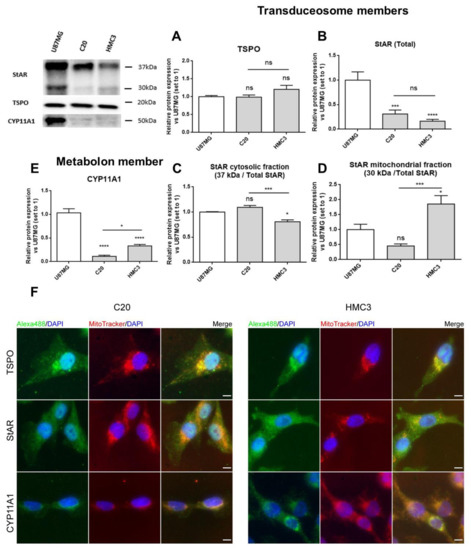
Figure 2. Expression of transduceosome/metabolon key proteins for neurosteroidogenesis in C20 and HMC3 cells. A representative Western blot panel for the expression of transduceosome members StAR and TSPO and the metabolon member CYP11A1 is reported. As shown in the panel, C20 and HMC3 cells expressed all transduceosome and metabolon members in basal conditions. (A–E) Densitometric analysis showed high expression of TSPO in both microglial cells (A), but StAR (B) and CYP11A1 (E) were expressed at lower levels if compared to the known neurosteroidogenic representative model (U87MG cells). Interestingly, the StAR cytosolic fraction (C) was reduced in HMC3 cells with respect to C20 cells. Moreover, the StAR mitochondrial fraction (D) and CYP11A1 in HMC3 cells seemed to be more expressed in comparison to C20 cells. Such differences could be due to the different developmental stages of the two microglial clones. Data are represented as means ± SEMs of three independent experiments. Statistical analysis was determined by one-way ANOVA followed by Bonferroni’s post-test: * p < 0.05, *** p < 0.001, **** p < 0.0001. (F) The expression of the transduceosome/metabolon members was also qualitatively evaluated with immunofluorescence analysis, which confirmed the Western blot results. The panel reports representative images of the co-localization study between TSPO, StAR, and CYP11A1 (green) with mitochondria stained with MitoTracker dye (red). DAPI staining is overlaid on both images to highlight cell nuclei. Scale bar: 10 μm.
The immunofluorescence analysis confirmed the presence of the investigated proteins and added details about their subcellular localization (Figure 2F). As expected, TSPO was detected mainly in the perinuclear region, as reported previously [39], and displayed good co-localization with mitochondria [42][43]. StAR appeared homogeneously distributed in cells, in agreement with its known dynamic shuttling between cytosol and mitochondria [20][23].The mitochondrial enzyme CYP11A1 presented perinuclear localization too, and the fluorescence intensity was higher in HMC3 than in C20 cells, in accordance with Western blot data (Figure 2E).
4. Discussion
Neuroactive steroids are the most potent endogenous modulators of microglial cells, and are capable of limiting their excessive reactivity, leading to beneficial effects in several neurodegenerative conditions. For several years there was widespread agreement that neurosteroidogenesis was not a specific function of microglial cells because in vitro and ex vivo models had evidenced gene transcription only for enzymes downstream the neurosteroidogenic cascade [31]. Thus, it has been commonly accepted that microglia lack de novo neurosteroidogenic capacity and have to be supplied with the first metabolites from other steroidogenic cell sources [31][32][33]. For this reason, the scientific community has paid poor attention to this biosynthetic process in microglia, while mainly investigating the effects induced by exogenously supplied neurosteroids [22]. This paradigm was questioned a few months ago following a study that has shown the ability of murine BV-2 microglial cells to produce de novo neurosteroids by the use of liquid chromatography with tandem mass spectrometry [34]. In the meantime, important results concerning this issue were obtained by our group in human microglia and reported in the present manuscript. Actually, little is known about human microglia, when also considering that substantial differences are emerging with respect to the murine-derived microglia [44][45]. Thus, it is of fundamental importance to deepen our knowledge about de novo neurosteroidogenesis in human microglia in order to draft a precise mechanistic model to direct new effective therapeutic interventions.
Herein, for the first time, the functional characterization of the neurosteroidogenic metabolic pathway was undertaken in two human microglial cell lines (C20 and HMC3), maintained in their resting condition. The results demonstrated that human microglia can produce and metabolize the first metabolite of neurosteroidogenesis, pregnenolone, suggesting that human microglia do have de novo neurosteroidogenic capacity. In line with this evidence, C20 and HMC3 cells constitutively expressed the key proteins of transduceosome/metabolon machinery that are notoriously required for the initiation of the biosynthetic process. Furthermore, they transcriptionally expressed the enzymes 3β−HSD and CYP17A1 that catalyze the conversion of pregnenolone to progesterone or DHEA, respectively.
The ability of human microglia to trigger neurosteroidogenesis indicated that the de novo production of neurosteroids appears to be required for the autocrine/paracrine regulation of the functionality of resting microglial cells. Indeed, starting from pregnenolone and progesterone, several other neurosteroids with peculiar effects can be produced [22]. Based on the cell type, different gene expression of the enzymes of the neurosteroidogenic cascade can direct the steroid metabolism. In both the investigated microglial clones, the enzymes 17β−HSD and 3β−HSD responsible for the production of androstenediol and progesterone were highly expressed. Conversely, both the microglial cells did not express the 5α-/5β-reductase, 3α-HSD, and aromatase enzymes, which are generally not required for activities under normal conditions. Notably, the androstenediol translation pathway has been implicated not only in the resolution of microglia-mediated inflammation, but also in the maintenance of normal CNS homeostasis by interacting with the ERβ receptor [35]. Progesterone is notoriously recognized to be a key steroid for regulating microglial activities involved in both the neuroinflammatory response and neuronal plasticity [46][47][48][49].
Several proteins are involved in steroid synthesis; among these, TSPO plays a pivotal role. TSPO binds cholesterol in coordination with the StAR protein, allowing the cholesterol translocation into mitochondria, which represents a crucial step of neurosteroidogenesis [20][23]. The high constitutive level of TSPO, observed in both the human microglial cells, suggested that this protein is important for maintaining the essential cellular activities. In resting human microglia, the crucial role of TSPO in mitochondrial metabolic activity has been recently documented [39]. Here, TSPO was found to have an important role in maintaining neurosteroidogenic efficiency in resting microglia. Indeed, following the TSPO silencing, reduced pregnenolone production was observed. However, the production of pregnenolone was not completely abolished, in line with previous findings [39].
In the past, results from studies involving TSPO knock-down or knock-out in vitro and in vivo models fueled doubts on TSPO involvement in the neurosteroidogenesis process [19][20]. However, with TSPO being highly conserved across all animal species and essential for cellular homeostasis [50][51], the possible establishment of compensatory mechanisms following TSPO silencing cannot be excluded. As reported in this work, silencing of this fundamental protein establishes a compensatory mechanism based on the increased expression of the mitochondrial StAR that probably compensates for the reduced cholesterol influx to the mitochondria due to the silencing of the TSPO gene. These data confirmed the results obtained by Fan et al., who observed increased expression of StAR protein in TSPO-deficient MA-10 cells [52]. The fact that the cells aim to restore this activity, when compromised, not only establishes the importance of TSPO in de novo neurosteroidogenesis, but also reinforces the key role of this metabolic pathway in maintaining the normal function of microglia. Noteworthily, the neurosteroidogenic efficiency allowed by the high levels of TSPO could be a pivotal requirement for the maintaining of the resting state. In support to this hypothesis, our previous results had shown that the TSPO silencing affects the microglial activation [14]. In particular, TSPO reduction has caused the imbalance of basally controlled production of anti-/pro-inflammatory cytokines favoring the release of the pro-inflammatory one. Future studies aimed at investigating the direct correlation of TSPO function with the increase of specific neurosteroids and the microglia activation state are certainly to be encouraged.
A result with potential therapeutic implications concerns the enhancement of neurosteroidogenic efficiency following the TSPO pharmacological stimulation that causes an increase in the cholesterol supply to CYP11A1. As previously reported, residence time (RT) is confirmed as a selective parameter for the prediction of the in vitro neurosteroidogenic efficacy of a synthetic TSPO ligand [53][54][55]. PIGA1138 [56] remains bound to TSPO for the longest time and demonstrated the highest pregnenolone production in C20 cells. Interestingly, some degree of PIGA1138 neurosteroidogenic capacity remained in the TSPO silenced C20 cells too. This effect could be explained by the long interaction of PIGA1138 with the residual amount of TSPO that remained following the silencing procedure. However, the possibility that the PIGA1138 effect was the consequence of its interaction with additional cellular targets involved in the neurosteroidogenesis modulation cannot be excluded. Precise experiments aimed at clarifying the events underlying such a phenomenon are certainly encouraged for future studies.
Noteworthily, the possibility to stimulate neurosteroidogenesis by TSPO ligands opens the way to deepening our knowledge on the precise contribution of neurosteroids directly produced by microglia in the autocrine regulation of homeostatic activities. From a therapeutic perspective, the results obtained offer the basis for considering de novo neurosteroidogenesis as a new strategic therapeutic target for the treatment of pathological conditions associated with dysregulations of reactive microglia [17]. Our previous results have already demonstrated the promising therapeutic potential of this target in the context of neuroinflammation. Indeed, highly steroidogenic TSPO ligands have induced the shift of the pro-inflammatory phenotype to the restorative one in human microglia. The phenotypic shift was abolished when the known inhibitor of CYP11A1 AMG was used [14][36].
The local autocrine and paracrine effects promoted by neurosteroids are broad and complex and could be classified as genomic and non-genomic [57]. The latter represent*s the most prominent ones due to the effects of neurosteroids on different receptors, in particular, GABAA and NMDA receptors [58][59]. Regarding genomic effects of neurosteroids, they are usually referred to as the action on gene expression of cytokines, chemokines and restorative factors, including neurotrophins, due to their effects on intracellular receptors, such as the receptors for progesterone, estrogen, androgens, and pregnane X. This work demonstrates for the first time a possible autocrine and genomic effect on the BDNF release mediated by the production of neurosteroids. BDNF represents a key neurotrophic factor responsible for neuronal survival whose levels have been shown to be reduced in several CNS pathologies [60][61][62][63]. Notably, the increase of BDNF release was linked to the pharmacological activation of TSPO. In this context, the extension of the analysis to other microglial neurotrophins, whose release can be promoted by the stimulation of the TSPO steroidogenic function, is certainly to be encouraged.
In conclusion, the demonstration that microglia can produce de novo neurosteroids suggests an important role of neurosteroidogenesis in the autocrine/paracrine modulation of microglia. This newly-demonstrated activity could represent a possible neuroprotective and modulatory mechanism of the CNS microenvironment by human microglia. The altered levels of neurosteroids in several CNS pathologies involving neuroinflammation confirm the implication of these molecules in tissue homeostasis. In this light, the high expression of TSPO in human microglial cells and the possibility of stimulating its steroidogenic function suggests this protein as a promising therapeutic target both for the control of excessive microglial activation and for the restoration of physiological neurosteroids levels.
References
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the Developing Nervous System. Trends Cell Biol. 2016, 26, 587–597.
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635.
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293.
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787.
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015, 7, 56.
- Franco, R.; Fernández-Suárez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86.
- Chen, Z.; Trapp, B.D. Microglia and neuroprotection. J. Neurochem. 2016, 136, 10–17.
- Liu, C.-Y.; Wang, X.; Liu, C.; Zhang, H.-L. Pharmacological Targeting of Microglial Activation: New Therapeutic Approach. Front. Cell. Neurosci. 2019, 13, 514.
- Yao, K.; Zu, H.-B. Microglial polarization: Novel therapeutic mechanism against Alzheimer’s disease. Inflammopharmacology 2020, 28, 95–110.
- Beckers, L.; Ory, D.; Geric, I.; Declercq, L.; Koole, M.; Kassiou, M.; Bormans, G.; Baes, M. Increased Expression of Translocator Protein (TSPO) Marks Pro-inflammatory Microglia but Does Not Predict Neurodegeneration. Mol. Imaging Biol. 2018, 20, 94–102.
- Guilarte, T.R. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol. Ther. 2019, 194, 44–58.
- Pannell, M.; Economopoulos, V.; Wilson, T.C.; Kersemans, V.; Isenegger, P.G.; Larkin, J.R.; Smart, S.; Gilchrist, S.; Gouverneur, V.; Sibson, N.R. Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia. Glia 2020, 68, 280–297.
- Liu, G.; Middleton, R.J.; Hatty, C.R.; Kam, W.W.; Chan, R.; Pham, T.; Harrison-Brown, M.; Dodson, E.; Veale, K.; Banati, R.B. The 18 kDa Translocator Protein, Microglia and Neuroinflammation. Brain Pathol. 2014, 24, 631–653.
- Da Pozzo, E.; Tremolanti, C.; Costa, B.; Giacomelli, C.; Milenkovic, V.M.; Bader, S.; Wetzel, C.H.; Rupprecht, R.; Taliani, S.; Da Settimo, F.; et al. Microglial Pro-Inflammatory and Anti-Inflammatory Phenotypes Are Modulated by Translocator Protein Activation. Int. J. Mol. Sci. 2019, 20, 4467.
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET Imaging as A Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161.
- Arbo, B.; Benetti, F.; Garcia-Segura, L.; Ribeiro, M. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J. Steroid Biochem. Mol. Biol. 2015, 154, 68–74.
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870.
- Barresi, E.; Robello, M.; Costa, B.; Da Pozzo, E.; Baglini, E.; Salerno, S.; Da Settimo, F.; Martini, C.; Taliani, S. An update into the medicinal chemistry of translocator protein (TSPO) ligands. Eur. J. Med. Chem. 2021, 209, 112924.
- Papadopoulos, V.; Fan, J.; Zirkin, B. Translocator protein (18 kDa): An update on its function in steroidogenesis. J. Neuroendocr. 2018, 30, e12500.
- Costa, B.; Da Pozzo, E.; Martini, C. Translocator protein and steroidogenesis. Biochem. J. 2018, 475, 901–904.
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.-Y.; He, X.-Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocr. 2016, 28, 12351.
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocr. 2019, 55, 100788.
- Costa, B.; Da Pozzo, E.; Martini, C. 18-kDa translocator protein association complexes in the brain: From structure to function. Biochem. Pharmacol. 2020, 177, 114015.
- Miller, W.L. StAR Search—What We Know about How the Steroidogenic Acute Regulatory Protein Mediates Mitochondrial Cholesterol Import. Mol. Endocrinol. 2007, 21, 589–601.
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2009, 1791, 646–658.
- De Nicola, A.F.; Garay, L.I.; Meyer, M.; Guennoun, R.; Sitruk-Ware, R.; Schumacher, M.; Deniselle, M.C.G. Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. J. Neuroendocr. 2018, 30, e12502.
- Lanussa, O.H.; Ávila-Rodriguez, M.; García-Segura, L.M.; González, J.; Echeverria, V.; Aliev, G.; Barreto, G.E. Microglial dependent protective effects of neuroactive steroids. CNS Neurol. Disord. Drug Targets 2016, 15, 242–249.
- Zwain, I.H.; Yen, S.S.C. Neurosteroidogenesis in Astrocytes, Oligodendrocytes, and Neurons of Cerebral Cortex of Rat Brain. Endocrinology 1999, 140, 3843–3852.
- Melcangi, R.C.; Azcoitia, I.; Galbiati, M.; Magnaghi, V.; Garcia-Ovejero, D.; Garcia-Segura, L.M. Non-neuronal cells in the nervous system: Sources and targets of neuroactive steroids. Gap Junctions 2003, 31, 535–559.
- Gago, N.; El-Etr, M.; Sananès, N.; Cadepond, F.; Samuel, D.; Avellana-Adalid, V.; Evercooren, A.B.-V.; Schumacher, M. 3α,5α-tetrahydroprogesterone (allopregnanolone) and γ-aminobutyric acid: Autocrine/paracrine interactions in the control of neonatal PSA-NCAM+progenitor proliferation. J. Neurosci. Res. 2004, 78, 770–783.
- Gottfried-Blackmore, A.; Sierra, A.; Jellinck, P.H.; McEwen, B.S.; Bulloch, K. Brain microglia express steroid-converting enzymes in the mouse. J. Steroid Biochem. Mol. Biol. 2008, 109, 96–107.
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319.
- Owen, D.R.; Narayan, N.; Wells, L.; Healy, L.; Smyth, E.; A Rabiner, E.; Galloway, D.; Williams, J.B.; Lehr, J.; Mandhair, H.; et al. Pro-inflammatory activation of primary microglia and macrophages increases 18 kDa translocator protein expression in rodents but not humans. Br. J. Pharmacol. 2017, 37, 2679–2690.
- Avallone, R.; Lucchi, C.; Puja, G.; Codeluppi, A.; Filaferro, M.; Vitale, G.; Rustichelli, C.; Biagini, G. BV-2 Microglial Cells Respond to Rotenone Toxic Insult by Modifying Pregnenolone, 5α-Dihydroprogesterone and Pregnanolone Levels. Cells 2020, 9, 2091.
- Saijo, K.; Collier, J.G.; Li, A.C.; Katzenellenbogen, J.A.; Glass, C.K. An ADIOL-ERβ-CtBP Transrepression Pathway Negatively Regulates Microglia-Mediated Inflammation. Cell 2011, 145, 584–595.
- Da Pozzo, E.; Giacomelli, C.; Costa, B.; Cavallini, C.; Taliani, S.; Barresi, E.; Da Settimo, F.; Martini, C. TSPO PIGA Ligands Promote Neurosteroidogenesis and Human Astrocyte Well-Being. Int. J. Mol. Sci. 2016, 17, 1028.
- Karri, S.; Dertien, J.S.; Stocco, D.M.; Syapin, P.J. Steroidogenic Acute Regulatory Protein Expression and Pregnenolone Synthesis in Rat Astrocyte Cultures. J. Neuroendocr. 2007, 19, 860–869.
- Liu, T.; Wimalasena, J.; Bowen, R.L.; Atwood, C.S. Luteinizing hormone receptor mediates neuronal pregnenolone production via up-regulation of steroidogenic acute regulatory protein expression. J. Neurochem. 2006, 100, 1329–1339.
- Milenkovic, V.M.; Slim, D.; Bader, S.; Koch, V.; Heinl, E.-S.; Alvarez-Carbonell, D.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. CRISPR-Cas9 Mediated TSPO Gene Knockout alters Respiration and Cellular Metabolism in Human Primary Microglia Cells. Int. J. Mol. Sci. 2019, 20, 3359.
- Yao, Y.; Fu, K.-Y. Serum-deprivation leads to activation-like changes in primary microglia and BV-2 cells but not astrocytes. Biomed. Rep. 2020, 13, 1.
- Costa, B.; Pini, S.; Gabelloni, P.; Da Pozzo, E.; Abelli, M.; Lari, L.; Preve, M.; Lucacchini, A.; Cassano, G.B.; Martini, C. The Spontaneous Ala147Thr Amino Acid Substitution within the Translocator Protein Influences Pregnenolone Production in Lymphomonocytes of Healthy Individuals. Endocrinology 2009, 150, 5438–5445.
- Rechichi, M.; Salvetti, A.; Chelli, B.; Costa, B.; Da Pozzo, E.; Spinetti, F.; Lena, A.; Evangelista, M.; Rainaldi, G.; Martini, C.; et al. TSPO over-expression increases motility, transmigration and proliferation properties of C6 rat glioma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2008, 1782, 118–125.
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. Int. J. Mol. Sci. 2017, 18, 786.
- Davis, R.L.; Buck, D.J.; McCracken, K.; Cox, G.W.; Das, S. Interleukin-1β-induced inflammatory signaling in C20 human microglial cells. Neuroimmunol. Neuroinflammation 2018, 2018, 50.
- Kaushik, D.K.; Thounaojam, M.C.; Kumawat, K.L.; Gupta, M.; Basu, A. Interleukin-1β orchestrates underlying inflammatory responses in microglia via Krüppel-like factor 4. J. Neurochem. 2013, 127, 233–244.
- Espinosa-Garcia, C.; Atif, F.; Yousuf, S.; Sayeed, I.; Neigh, G.N.; Stein, D.G. Progesterone Attenuates Stress-Induced NLRP3 Inflammasome Activation and Enhances Autophagy Following Ischemic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3740.
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Boroujeni, F.B.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139.
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr. Neuropharmacol. 2016, 14, 641–653.
- Wong, A.M.; Rozovsky, I.; Arimoto, J.M.; Du, Y.; Wei, M.; Morgan, T.E.; Finch, C.E. Progesterone Influence on Neurite Outgrowth Involves Microglia. Endocrinology 2008, 150, 324–332.
- Fan, J.; Campioli, E.; Midzak, A.; Culty, M.; Papadopoulos, V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc. Natl. Acad. Sci. USA 2015, 112, 7261–7266.
- Fan, J.; Campioli, E.; Sottas, C.; Zirkin, B.; Papadopoulos, V. Amhr2-Cre–Mediated Global Tspo Knockout. J. Endocr. Soc. 2020, 4, bvaa001.
- Fan, J.; Wang, K.; Zirkin, B.; Papadopoulos, V. CRISPR/Cas9‒Mediated Tspo Gene Mutations Lead to Reduced Mitochondrial Membrane Potential and Steroid Formation in MA-10 Mouse Tumor Leydig Cells. Endocrinology 2018, 159, 1130–1146.
- Costa, B.; Da Pozzo, E.; Cavallini, C.; Taliani, S.; Da Settimo, F.; Martini, C. Long Residence Time at the Neurosteroidogenic 18 kDa Translocator Protein Characterizes the Anxiolytic Ligand XBD173. ACS Chem. Neurosci. 2016, 7, 1041–1046.
- Costa, B.; Da Pozzo, E.; Giacomelli, C.; Barresi, E.; Taliani, S.; Da Settimo, F.; Martini, C. TSPO ligand residence time: A new parameter to predict compound neurosteroidogenic efficacy. Sci. Rep. 2016, 6, 18164.
- Costa, B.; Cavallini, C.; Da Pozzo, E.; Taliani, S.; Da Settimo, F.; Martini, C. The Anxiolytic Etifoxine Binds to TSPO Ro5-4864 Binding Site with Long Residence Time Showing a High Neurosteroidogenic Activity. ACS Chem. Neurosci. 2017, 8, 1448–1454.
- Barresi, E.; Bruno, A.; Taliani, S.; Cosconati, S.; Da Pozzo, E.; Salerno, S.; Simorini, F.; Daniele, S.; Giacomelli, C.; Marini, A.M.; et al. Deepening the Topology of the Translocator Protein Binding Site by Novel N,N-Dialkyl-2-arylindol-3-ylglyoxylamides. J. Med. Chem. 2015, 58, 6081–6092.
- Balthazart, J.; Choleris, E.; Remage-Healey, L. Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Horm. Behav. 2018, 99, 1–8.
- Tuem, K.B.; Atey, T.M. Neuroactive Steroids: Receptor Interactions and Responses. Front. Neurol. 2017, 8, 442.
- Giatti, S.; Garcia-Segura, L.M.; Barreto, G.E.; Melcangi, R.C. Neuroactive steroids, neurosteroidogenesis and sex. Prog. Neurobiol. 2019, 176, 1–17.
- Wang, T.; Ye, X.; Bian, W.; Chen, Z.; Du, J.; Li, M.; Zhou, P.; Cui, H.; Ding, Y.-Q.; Qi, S.; et al. Allopregnanolone Modulates GABAAR-Dependent CaMKIIδ3 and BDNF to Protect SH-SY5Y Cells Against 6-OHDA-Induced Damage. Front. Cell. Neurosci. 2020, 13, 569.
- Meyer, M.; Garay, L.I.; Kruse, M.S.; Lara, A.; Gargiulo-Monachelli, G.; Schumacher, M.; Guennoun, R.; Coirini, H.; De Nicola, A.F.; Deniselle, M.C.G. Protective effects of the neurosteroid allopregnanolone in a mouse model of spontaneous motoneuron degeneration. J. Steroid Biochem. Mol. Biol. 2017, 174, 201–216.
- Nin, M.S.; Martinez, L.A.; Pibiri, F.; Nelson, M.; Pinna, G. Neurosteroids reduce social isolation-induced behavioral deficits: A proposed link with neurosteroid-mediated upregulation of BDNF expression. Front. Endocrinol. 2011, 2, 73.
- Almeida, F.B.; Gomez, R.; Barros, H.M.T.; Nin, M.S. Hemisphere-dependent Changes in mRNA Expression of GABAA Receptor Subunits and BDNF after Intra-prefrontal Cortex Allopregnanolone Infusion in Rats. Neuroscience 2019, 397, 56–66.




