
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Felisa Reyes-Ortega | + 4272 word(s) | 4272 | 2021-01-27 04:32:35 | | | |
| 2 | Peter Tang | Meta information modification | 4272 | 2021-04-30 09:57:27 | | |
Video Upload Options
Biofilm-associated infections pose a huge burden on healthcare systems worldwide, with recurrent lung infections occurring due to the persistence of biofilm bacteria populations. In cystic fibrosis (CF), thick viscous mucus acts not only as a physical barrier, but also serves as a nidus for infection. Increased antibiotic resistance in the recent years indicates that current therapeutic strategies aimed at biofilm-associated infections are “failing”, emphasizing the need to develop new and improved drug delivery systems with higher efficacy and efficiency. Magnetic nanoparticles (MNPs) have unique and favourable properties encompassing biocompatibility, biodegradability, magnetic and heat-mediated characteristics, making them suitable drug carriers. Additionally, an external magnetic force can be applied to enhance drug delivery to target sites, acting as “nano-knives”, cutting through the bacterial biofilm layer and characteristically thick mucus in CF.
1. Introduction
More than 60% of all bacterial infections are associated with biofilm development [1][2]. Biofilms can occur not only in chronic wound infections, but also in exogenous medical devices, such as catheters, implants and prosthesis [1][2]. Biofilms are typically defined as complex communities of surface-associated bacteria encased in a self-produced extracellular polysaccharide matrix that are adherent to biological or abiotic surfaces [3]. Traditional treatment approaches for biofilms typically involve the use of conventional antibiotics with bactericidal (bacterial killing) or bacteriostatic (inhibition of bacterial dividing) properties [2]. To effectively eradicate biofilms, the antibiotics have to penetrate the bacterial cell membrane and accumulate to reach therapeutic concentrations [2]. Unfortunately, in most scenarios, conventional antibiotics are not able to completely eradicate biofilms due to the unique structure and properties of the biofilm (Figure 1).
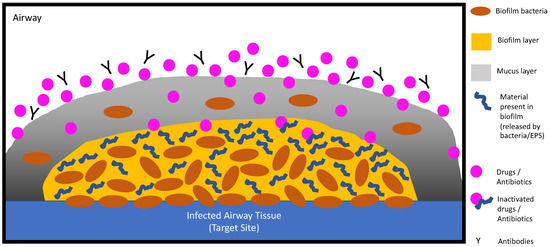
Figure 1. Current therapeutics used are ineffective in penetrating the biofilm layer to reach the target site. In cystic fibrosis (CF), this is further hindered with an additional layer of thick, viscous mucus.
Opposite to planktonic bacteria which can be easily removed with gentle rinsing, biofilm bacteria adhere onto surfaces. This offers the biofilm colony unique properties such as protecting inner isolates, promoting survival in hostile environments and conferring resistance to antimicrobial agents and the host immune system, resulting in persistent and chronic infections and hence, are 1000-fold more tolerant to antibiotics [2][4][5]. This tolerance offers an explanation as to why current antibiotic treatments are mostly ineffective against biofilm-associated infections and why patients with biofilm infections often suffer from chronic complications [6]. As previous strategies to treat biofilm infections have not been shown to be as clinically successful as hoped for, there is an unmet medical need that necessitates the development of alternative methods, including novel antibacterial agents and improved drug delivery systems, to avoid problems associated with short half-lives, low bioavailability or systemic toxicity.
In recent years, magnetic nanoparticles (MNPs) have become increasingly popular as drug delivery systems in diagnosis, e.g., imaging contrast agent, and in therapy, e.g., magnetic hyperthermia combining pharmaceutical agents and drug delivery as carriers themselves [2]. The effectiveness of MNPs can be measured by parameters such as administration method, particle size, in vivo drug release kinetics, drug loading and intrinsic carrier toxicity [2]. Furthermore, delivery with MNPs allows for pre-determining drug kinetics and targets which are essential in maintaining optimal dosing within the therapeutic window using a lower amount of drug, and therefore decreasing the toxicity and cost of the pharmaceutical formulation. Moreover, MNPs can be administered via a plethora of different routes ranging from local to systemic administrations to achieve targeted and improved delivery of pharmaceuticals, potentially improving bioavailability or allowing for sustained drug release or prolonged drug exposure. In patients suffering from cystic fibrosis (CF), MNPs have been shown to significantly improve patient outcomes as these encapsulated antibiotics were able to be transported through the sticky mucus barrier in CF lungs [2][7].
2. Biofilm Formation and Resistance
2.1. Biofilm Development Cycle
There are five stages that characterise the formation of a three-dimensional (3D) complex biofilm structure (Figure 2).
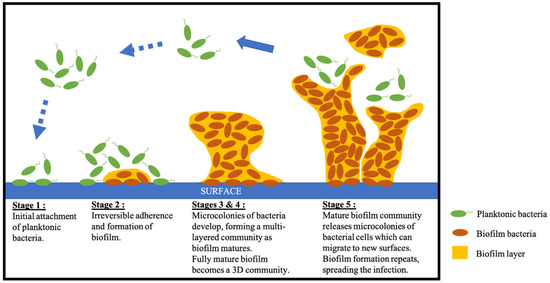
Figure 2. Schematic of the five stages of biofilm development including initiation, adherence and formation, development of mature biofilm and biofilm spreading.
In the first stage, individual planktonic cells adhere to a surface and initiate the production of biofilm by encasing protective exopolymeric material in small quantities [6]. In the second stage, adherent cells exude an extracellular polymeric substance (EPS) which promotes irreversible attachment of cells to the surface, cell aggregation and the formation of the biofilm matrix [6]. In the third step, micro-colonies and water channel architecture develop, which significantly enhances nutrient supply, maturing the layered biofilm [6]. In the fourth stage, cell density reaches its capacity, and the fully mature biofilm is now considered a three-dimensional community [6]. In the final stage, micro-colonies are released from the mature biofilm community which can migrate to new surfaces and initiate the formation of new biofilm colonies, resulting in spreading of the infection [6][8].
2.2. Resistance
The rise of antibiotic resistance poses a tremendous and increasing burden on the global healthcare system, with biofilms playing a large contributory role to this health crisis [2]. In addition, the aforementioned contributing factors associated with the structure and physiological attributes of biofilm organisms also confer an inherent resistance to antimicrobial agents, resulting in chronic infections [9].
In early studies, it was originally postulated that biofilm matrixes could serve as a physical barrier preventing the diffusion of antibiotics; however, more recent studies disproved this hypothesis, showing that antibiotic diffusion is not hindered by biofilm matrices [10]. It is more likely that either drug penetration is only restricted if antibiotics bind to biofilm components such as deoxyribonucleic acids (DNA), proteins or polysaccharides, or that antibiotics are inactivated or are unable to reach therapeutic concentrations when reaching the target site [2][10]. Additional factors influencing antibacterial activity are that bacteria grown in biofilms display lower metabolic activities, resultant of limited oxygen and nutrient access, which promotes tolerance to antibiotics targeting replication or biosynthesis of bacterial cell walls [10].
Moreover, hyper-mutability of isolates such as Pseudomonas aeruginosa (P. aeruginosa) growing in biofilms promotes the emergence of resistance mutations which are selected for under the pressure of repeated courses of antibiotics, which is often the case in infections associated with CF [11]. P. aeruginosa colonies can also form tall ridges or wrinkles which facilitates oxygen supply, further promoting survival and growth [12][13]. With this in mind, we review herein the various MNP strategies to treat biofilm infections caused by CF-relevant pathogens (Table 1). Further sub-populations of persister cells, which are slow or non-dividing bacterial cells, are able to survive antibiotics targeting fundamental cellular processes such as cell replication or cell wall synthesis [10]. These persister cells can prove to be very problematic as they can become dormant as a result of bacterial differentiation, and consequently contribute to incomplete bacteria killing by therapeutic interventions [2][10].
Table 1. Summary of CF-related in vitro biofilms treated with antibiotic + nanoparticles combinations.
|
Isolate |
NP Component |
Antibiotic Component |
Size (nm) |
Biofilm Effect |
Reference |
||
|
Inhibition |
Disruption |
Viability |
|||||
|
Pseudomonas aeruginosa |
Chitosan |
Erythrosine |
80.9 ± 7.43 |
- |
- |
78% |
[14] |
|
Chitosan capped Silver |
Aztreonam |
10 |
- |
98% |
0% |
[15] |
|
|
Silver made from Allophylus cobbe |
Ampicillin |
5.0 ± 4.0 |
69% |
- |
- |
[16] |
|
|
Silver made from Allophylus cobbe |
Vancomycin |
5.0 ± 4.0 |
54% |
- |
- |
[16] |
|
|
PLGA nanoparticles coated with PL and DNAse 1 |
Ciprofloxacin |
251.9 |
100% |
95% |
- |
[17] |
|
|
PLGA |
Ciprofloxacin and MNP |
220.9 ± 7.4 |
- |
- |
67% |
[18] |
|
|
PLGA |
Gentamycin |
241.3 ± 12.4 |
- |
- |
3% |
[19] |
|
|
PLGA, chitosan |
Colistin |
300 |
- |
50% |
|
[20] |
|
|
PLGA, phosphatidylcholine |
Levofloxacin |
240 ± 50 |
- |
- |
5–19% |
[21] |
|
|
Staphylococcus aureus |
Silver made from Allophylus cobbe |
Ampicillin |
5 ± 4 |
49% |
- |
- |
[16] |
|
Silver made from Allophylus cobbe |
Vancomycin |
5 ± 4 |
73% |
- |
- |
[16] |
|
Adapted from Han et al. [2].
2.3. Magnetic Nanoparticles in Biofilm Treatment
The application of MNPs in the diagnosis and treatment of diseases such as cancer has grown exponentially in the last decade, and this immense wealth of knowledge can be used to design novel biofilm treatment formulations and drug delivery carriers for respiratory diseases [22]. Additionally, the symbiotic marriage of nanopharmacy and targeted drug delivery has resulted in several nanoscale designs of magnetic materials as ideal drug delivery carriers [23]. The most commonly used MNPs have low toxicity, and employ ferromagnetic elements such as cobalt, iron, nickel and their Food and Drug Administration (FDA)-approved compound ferrites, magnetite (Fe3O4) and maghemite (Fe2O3), that can be easily synthesized with a controlled and precise shape and size [23][24][25].
MNPs have also been studied deeply as potential and useful bacterial detection and bacterial separation agents due to their magnetic properties and antimicrobial effect. These MNPs are usually coated with lipids, polymers or silica in order to make them biocompatible and are being utilised as drug delivery carriers. Some previous studies demonstrated that magnetic iron oxide nanoparticles under the application of a magnetic field were able to promote an antimicrobial effect in biofilm matrixes, causing detachment of several bacteria, such as Methicillin-resistant Staphylococcus aureus (MRSA) [26]. MNPs have also been demonstrated to be good contrast agents for in vivo bacterial imaging due to their superparamagnetic properties, apart from enhancing antimicrobial efficacy, protecting antimicrobial agents from deactivating enzymes and releasing antimicrobial agents in a sustained and controlled manner, improving bioavailability and reducing systemic side effects [2]. The interactions of MNPs and biofilm can be summarised as a three-step process: (1) transportation of MNPs to the biofilm, (2) attachment to the biofilm surface and (3) MNP migration within the biofilm [27]. A complex interplay of factors such as, but not limited to, MNP characteristics, biofilm matrix biological and physiochemical makeup, as well as environmental parameters, occurs at each step [27]. Additionally, the nanoscale dimension of MNPs, ranging from 1 to 100 nm, offers unique properties such as large surface area to volume ratio, versatility and surface charge, all of which can enhance MNPs’ influence on other microorganisms [5]. The advantages of utilising these MNP formulations over traditional/conventional systems are discussed below.
2.4. Cystic Fibrosis Infections and Biofilms
CF is an inherited chronic disease characterised by recurring infections and inflammation in the lower respiratory system [28]. Due to the defective CF transmembrane conductance regulator (CFTR) protein chloride, sodium and bicarbonate transport is impaired, which results in viscous mucus and impairs mucociliary clearance, promoting airway colonisation by bacteria [9][29][30]. In paediatric patients, most commonly, bacterial populations include Burkholderia cepacian, Haemophilus influenzae (H. influenzae) and Staphylococcus aureus (S. aureus). Pseudomonas aeruginosa (P. aeruginosa) infections are the most common in adult CF patients and chronic colonisation is directly correlated with worse patient outcomes [29]. This correlation is likely due to the fact that these infections are caused by biofilm-producing isolates [9][29]. In CF, P. aeruginosa typically begins as a non-mucoid phenotype, however, persistence of the organism in CF lungs result in a shift towards a mucoid phenotype that produces alginate, a polysaccharide material characteristic of P. aeruginosa biofilm infections [9]. Large amounts of exopolysaccharide alginate cause the mucus to be extra viscous, promoting and supporting the formation of biofilms in the lungs [9]. Additionally, the alginate layer of mucoid strains can prevent antibody coatings and block immunological determinants necessary for opsonic phagocytosis [9]. Mucoid strains have also been reported to be more resistant to non-opsonic phagocytosis than non-mucoid strains, and the presence of alginate can promote adherence of mucoid strains to pulmonary tract epithelial cells, thus inhibiting clearance [9]. Furthermore, the sputum from CF patient lungs is usually filled with large numbers of polymorphonuclear leukocytes (PMN) and the host inflammatory defence mechanisms against mucoid P. aeruginosa are dominated by PMNs, which can produce oxygen radicals that consequently induce mutations, leading to more alginate production [9]. Hence, there is a major unmet medical need for effective antibiotic therapies to treat chronic lung infections in CF patients. It is paramount that next-generation therapies not only target non-biofilm forming isolates but are able to eradicate complex biofilm-forming isolates.
2.5. Current Antibiotic-MNP Treatment Options for CF Biofilms
Once chronic P. aeruginosa infection occurs in CF lungs, long-term maintenance therapy with inhaled therapeutics is typically used to suppress infection and maintain lung function [29]. Three commonly used antibiotics are tobramycin, aztreonam and colistinmethate sodium. Tobramycin has been shown to improve the eradication of planktonic P. aeruginosa by conjugating with carboxyl groups of citric acid-coated MNPs [31]. Aztreonam is a monocyclic β-lactams antibiotic that is typically used against Gram-negative bacteria by inhibiting cell wall synthesis [32]. When used in combination with silver NPs, P. aeruginosa biofilm thickness and biomass were reduced by 50% and up to 98%, respectively [15]. Colistinmethate sodium is a last-line antibiotic against Gram-negative pathogens and is typically used to treat chronic endobronchial P. aeruginosa infections in CF patients [33].
One of the greatest challenges in using MNPs to treat CF is the successful penetration of the characteristically thick and sticky mucus, which reduces drug delivery efficacy and sterically hinders drug delivery to the lung [34]. The small size of MNPs make them promising vehicles to package and transport drugs through the mucus [34]. More efficient penetration of the mucus by MNPs can also be achieved by attaching mycolytics onto MNPs or eliminating potential electrostatic interactions of the MNPs with the mucus by coating MNPs with an electrostatically neutral material such as polyethylene glycol (PEG) (Figure 3) [34].
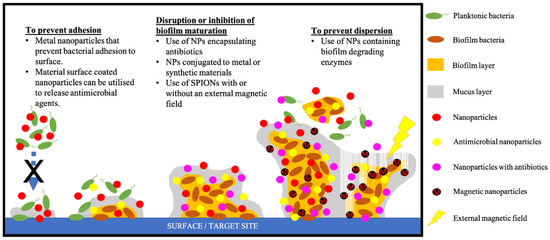
Figure 3. Nanoparticle-based strategies that can be used to target each stage of biofilm bacteria development.
3. Approaches for Prevention and Treatment
3.1. Inorganic Metal NPs
Metallic NPs such as copper, silver and gold have been found to possess strong antimicrobial activity [35]. Their clinical application was originally slowed down by potential toxicity to mammalian cells, however, due to increasing concerns about antibiotic resistance of biofilms and bacterial infections, these MNPs have once again reignited interest in their application (Figure 4) [35].
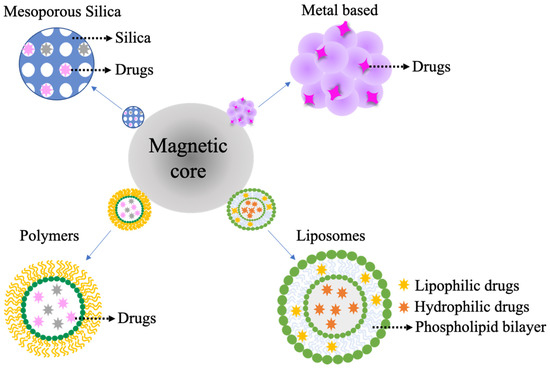
Figure 4. Types of nanomaterials that can be added to a magnetic nanoparticle core for more efficient and targeted drug delivery.
3.2. Advantages of Utilising MNPs over Traditional/Conventional Systems
MNPs convert magnetic energy into thermal energy under the influence of an external magnetic field, essentially acting as heat mediators [34]. In hyperthermia, upon the application of an external magnetic field, the alternative magnetic field (AMF) oscillates at a speed faster than the relaxation time of MNP, resulting in a delay of magnetic moment relaxation, generating heat [34]. MNPs then mediate heat via relaxation losses through Neel relaxation, which reorients the magnetic moment in the same direction as the applied magnetic field, and Brownian relaxation, a resultant friction force from the rotation of NPs in a liquid suspension [34]. Neel relaxation is size-dependent and prevails only in small NPs, while Brownian relaxation is size- and viscosity-dependent [34]. Intensive efforts have been centred on developing optimal synthesis protocols of uniform and reproducible MNPs that have well-defined shape and size with superparamagnetic behaviour [34]. Under the influence of an external magnetic force, superparamagnetism allows MNPs to deliver drugs specifically to the target site with minimal exposure to other healthy cells [34]. Importantly, in the treatment of CF, MNPs under the application of an external magnetic force can act as nano-knives (Figure 3), penetrating the thick mucus and biofilm layers, exposing the inner layer of microorganisms to delivered drugs at the target site [36]. Moreover, lungs are filled with air, a poor conductor of heat, thus, heat generated by the MNPs in the lung is unlikely to affect surrounding organs such as the heart [34].
Taken together, the properties of MNPs make them an ideal and effective approach for drug delivery.
3.3. Toxicity vs. Efficacy of MNPs in Biomedical Applications
MNPs have been extensively studied to prove their biocompatibility and toxicity in humans. In general, the distribution of MNPs inside the body depends on various factors, such as dose, particle size, chemical nature, surface area, reactivity, charge, ease to aggregation and duration of exposure, which also affects the potential toxic effects [37][38]. Knowledge about their toxicity and health impact is essential before these nanomaterials can be used in clinical trials. Most of the MNPs applied in biomedical applications are previously coated with liposomes, silica or biopolymers to make them biocompatible and to decrease their toxicity. These coatings also allow for the modification of surface charge nanoparticle, that can be adjusted to facilitate MNPs elimination from the body [39]. Biopolymers most frequently used with this purpose are PEG [40], poly-d-l-(lactic-co-glycolic acid) (PLGA) [41], polyactic acid (PLA), poly(caprolactone) (PCL) [42], poly(dimethyl aminoethyl methacrylate) (PDMAEMA) [43], Poly(N-isopropylacrylamide) (PNIPAM) [44], Dextran [45], Chitosan [46], poly(urethanes) (PUs) [47] and poly(ethyelene imine) (PEI) [48]. All of these polymers can undergo degradation processes to be broken down into products that can be safely processed in the body.
Biocompatibility and ease of elimination from the human body are major concerns when using MNPs. Many studies show MNPs as safe nanostructures that can be applied in biomedical applications, with a small handful demonstrating side effects related to the use of these MNPs. For example, numerous studies have been carried out with gold nanoparticles to demonstrate their safety for biological systems. Bailly et al. showed that gold NPs conjugated with dextran were rapidly eliminated from the blood circulation and accumulated preferentially in the liver and spleen without inducing liver or kidney toxicity [49]. The authors also studied the effect of residual accumulation of these NPs in tissues. Not only did they not detect any sign of histological damage or inflammation in tissues, they also confirmed the absence of any chronic inflammation in the animals studied. Abedin et al. demonstrated that gold nanoparticles coated with poly-l-lysine increased stability in vivo due to the polymer layer [50]. On the other hand, Korani et al. studied the effect of silver nanoparticles in human health and showed a dose-dependent toxic response in several organs [51]. Munger et al. carried out a controlled, cross-over time exposure study of commercial silver nanoparticles, demonstrating the absence of any changes in human metabolic, hematologic, urine and physical findings or imaging morphology [52]. Balfourier et al. showed that gold nanoparticles could be metabolised by mammal cells, bringing an insight on the elimination of gold nanoparticles from organisms [53]. On the other hand, Talapko et al. have demonstrated that silver nanoparticles could originate methemoglobin, localised argyria and systemic argyria [54], Thapa et al. showed that gold nanoparticles could lead to fibroblast cell cytotoxicity and inflammatory responses [55] and Arvizo et al. showed that gold nanocomplexes showed poor biocompatibility and were not easy to biodegrade into the organism [56].
Consequently, it is clear that MNPs used in biomedical applications, specifically as anti-biofilm therapy, require several physicochemical characterisations to ensure that size, chemical nature, surface area, exposure time, reactivity, charge and compositions are suitable to avoid toxicity and aggregation. It is also noteworthy that most of the research described show in vitro and in vivo data, or describe animal studies, with limited research conducted in humans. Therefore, it is highly recommended to focus future research on clinical trials to better understand the risk of MNPs in humans.
3.4. MNPs Coated by Liposomes
Liposomes are amphiphilic phospholipids that readily form spherical lipid vesicles ranging from 20 nm to 20 µm [32]. A major advantage is that liposomes are biocompatible, and depending on the number of lipid bilayers, are classified as unilamellar or multilamellar [2]. Due to amphiphilic properties, liposomes have high drug-carrying capacity, making them ideal vehicles for larger drugs such as antibiotics. Furthermore, liposomes can be used to encapsulate magnetic cores within its hydrophilic characteristic core, or hydrophobic compounds within the bilayer [2]. The bilayer membrane structure also allows for greater permeability, facilitating cellular uptake of liposomes (Figure 4). In addition, liposomal encapsulation of the hydrophilic core offers hydrophilic compounds protection from deactivating factors in vivo [2].
Advantages of using magnetic core-liposome nanoparticles include the incorporation of a number of drugs, including antibiotics offering protection from enzymatic inactivation and degradation, as well as the ability to overcome drug resistance of extracellular pathogens [32][57]. Additionally, parameters such as lipid composition, charge and size magnetic liposome can be altered, allowing flexible and fit-to-purpose drug-carrying capacity and drug release rate [32][58].
As CF biofilms are typically surrounded by a thick and sticky mucus layers, sufficient drug delivery to affected sites is crucial. Targeted delivery such as in CF biofilms can be achieved through size-dependent penetration. Dong et al. found that small sized, cationic unilamellar particles ranging from 128 to 141 nm were able to penetrate S. aureus and P. aeruginosa biofilms more deeply and at a higher rate than larger multilamellar particles [59]. The smaller cationic liposomes also inhibited biofilm growth of P. aeruginosa and S. aureus by 75% and 43%, respectively [59]. Additionally, the authors hypothesised that the electrostatic equilibrium of colonies within the biofilm might have been disrupted by cationic unilamellar particles, further enhancing its anti-biofilm effects [59]. Unfortunately, increased drug activity of cationic liposomes also shows amplified toxicity to human lung cells compared to negatively charged or uncharged systems. This highlights the intricate harm–benefit balance caused by different nanoparticle synthesis or surface charges [7].
As low concentrations of compounds to biofilm infection sites are rapidly cleared from the lungs, a number of studies have investigated the drug delivery of liposomes to the lungs via inhalation as liquid or dry formulations [60]. Joshi et al. describe a dry powder liposome-based anti-inflammatory corticosteroid formulation maintaining desired drug concentrations over a prolonged period of time [61]. Meers et al. investigated the efficacy of amikacin as a liposomal formulation compared to tobramycin for the treatment of CF. The authors found that 65% of amikacin remained in liposomal form after deposition in the lung compared to free tobramycin in rats [62]. Additionally, free tobramycin was completely removed from the lung within 2 h, while liposomal amikacin demonstrated a sustained drug release up to 175 h following a burst release which transformed liposomal amikacin into free form during inhalation [62]. Despite these promising initial results, clinical applications of liposomal formulations are currently limited by poor physiochemical properties. 35% of amikacin did not reach the lungs in liposomal form, and leakage of encapsulated antibiotics from liposomes might result in unfavourable side effects [62]. Despite promising potential for pulmonary drug administration, effective drug delivery remains a challenge for liposomal formulations.
3.5. MNPs Coated by polymers
3.5.1. Chitosan
Chitosan is a biodegradable, biocompatible, natural polymer which possesses inherent broad-spectrum antimicrobial properties [2]. As chitosan NPs have a positive surface charge, they are expected to have higher affinities to negatively charged biofilm-affected areas compared to negatively charged NPs [2]. Additionally, the high surface area to volume ratio of nanosized particles can enhance the antimicrobial activity of chitosan NPs. It has been hypothesised that chitosan NPs interact with bacteria through electrostatic interactions, inducing cell permeability changes and resulting in cell morphology modifications, which subsequently lead to leakage of internal proteins and DNA, culminating to bacterial cell death [63][64].
Previous studies have shown that chitosan nanoparticles are effective in inhibiting cell growth of various bacterial isolates [65][66][67]. Similar to other vehicles, chitosan MNPs can be loaded with antibiotics to enhance the effectiveness of the respective compound [68][69]. The aminoglycoside antibiotic gentamicin has low bioavailability and short half-life, which often requires high doses, potentially leading to increased drug resistance [70]. Wang et al. reported that gentamicin-loaded chitosan MNPs were able to achieve deeper penetration of mature S. aureus biofilm by application with a magnetic field, resulting in increased antibiotic delivery and effectiveness for biofilm eradication [69]. Other studies corroborate that the use of an external magnetic force is able to achieve higher penetration of MNPs into the biofilm structure, allowing for more efficient biofilm bacteria eradication [71][72]. Taken together, these findings highlight the promising potential of utilizing chitosan-based MNPs to enhance the effectiveness of antibiotics with precision through the use of an external magnetic field while circumventing potential toxicity or low bioavailability issues.
3.5.2. Poly-d-l-(Lactic-Co-Glycolic Acid) (PLGA)
The aforementioned limitation of sufficient lung delivery is particularly challenging in CF. Different attempts have been made with strategies using NPs coated with metal or liposomal materials, as well as embedded with antibiotics. In recent times, synthetic polymers such as the FDA-approved PLGA have increasingly garnered more attention due to their favourable biocompatibility and biodegradability properties [32].
PLGA NPs have highly desirable versatile physiochemical properties that allow for easy composition and degradation modifications as well as offering a wide variety of design and development of novel materials. Strategies using PLGA NPs encapsulated with antibiotic ciprofloxacin were found to have high drug loading against P. aeruginosa strains and exhibited good mucus penetration due to its nanometric size and surface properties [73][74]. Enhanced antimicrobial activity with lower ciprofloxacin dosages were also reported [73]. Thomas et al. employed PEG-coated PLGA nano- and micro-particles encapsulating tobramycin that exhibited enhanced antimicrobial activity and good penetration of mucus and bacterial biofilm [75]. Furthermore, more opportunities for triggered release properties and increased targeting efficacy arise from incorporating superparamagnetic particles into PLGA NPs. This approach was employed in a study where ciprofloxacin and MNPs were encapsulated within PLGA NPs [76]. The authors report that the rate of drug release surged when an external magnetic field was applied, and plateaued in the absence of the magnetic field, indicating its potential for controlled drug release [76].
Taken together, these studies highlight the advantages of using PLGA NPs’ versatility and biocompatibility in combination with magnetic nanoparticles for a controlled and triggered drug delivery system that can be used for efficient antimicrobial treatment in CF.
3.6 MNPs Coated by Silica
Silica-coated MNPs are strong, biocompatible compounds, albeit not biodegradable. However, their unique composition allows for overcoming some of the challenges of organic biodegradable NPs, such as releasing of active agents when in contact with water, premature leakage of encapsulated materials, or a burst release when attached to NP surfaces [2][77]. Silica NPs are typically paired as a carrier with nitric oxide (NO), an endogenous free radical involved in several biological processes [2]. NO, as a carrier, offers unique benefits such as its own broad-spectrum antimicrobial property and low toxicity, and together with its by-products, can eradicate biofilms [2]. A number of independent laboratories have reported that Gram-negative biofilms are more susceptible to NO-releasing silica NPs than Gram-positive biofilms [78][79]. When comparing silica NPs of varying shapes and sizes for the treatment of P. aeruginosa and S. aureus biofilms, Hetrick et al. observed that small (14 nm) and rod-like NPs were the most effective biofilm bacterial killers [79]. The 14 nm NPs were able to diffuse into the biofilm more quickly than larger 150 nm NPs, dispersing the biofilm within minutes, resulting in bacterial cell death [79]. Additionally, rapid release of NO in high concentrations from small silica NPs < 20 nm indicated promising potential in eradicating Gram-negative biofilms [79]. Short treatment durations of 15 min with rod-like NPs were effective in killing bacterial cells and dispersed the biofilm after 60 min. Interestingly, spherical NPs were confined to a limited area in the biofilm, and bacterial cell death was only observed after 60 minutes, highlighting the importance of size and shape [79].
References
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11.
- Han, C.; Romero, N.; Fischer, S.; Dookran, J.; Berger, A.; Doiron, A. Recent Developments in the use of Nanoparticles for Treatment of Biofilms. Nanotechnol. Rev. 2017, 6, 383–404.
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322.
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281.
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806.
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824.
- d’Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 92–111.
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187.
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193.
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319.
- Schneider-Futschik, E.K.; Paulin, O.K.A.; Hoyer, D.; Roberts, K.D.; Ziogas, J.; Baker, M.A.; Karas, J.; Li, J.; Velkov, T. Sputum Active Polymyxin Lipopeptides: Activity against Cystic Fibrosis Pseudomonas aeruginosa Isolates and Their Interactions with Sputum Biomolecules. ACS Infect. Dis. 2018, 4, 646–655.
- Kawano, Y.; Jordan, O.; Hanawa, T.; Borchard, G.; Patrulea, V. Are Antimicrobial Peptide Dendrimers an Escape from ESKAPE? Adv. Wound Care 2020, 9, 378–395.
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9.
- Chen, C.P.; Chen, C.T.; Tsai, T. Chitosan nanoparticles for antimicrobial photodynamic inactivation: Characterization and in vitro investigation. Photochem. Photobiol. 2012, 88, 570–576.
- Habash, M.B.; Park, A.J.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Synergy of silver nanoparticles and aztreonam against Pseudomonas aeruginosa PAO1 biofilms. Antimicrob. Agents Chemother. 2014, 58, 5818–5830.
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344.
- Baelo, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 2015, 209, 150–158.
- Hua, X.; Tan, S.; Bandara, H.M.H.N.; Fu, Y.; Liu, S.; Smyth, H.D.C. Externally Controlled Triggered-Release of Drug from PLGA Micro and Nanoparticles. PLoS ONE 2014, 9, e114271.
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063.
- d’Angelo, I.; Casciaro, B.; Miro, A.; Quaglia, F.; Mangoni, M.L.; Ungaro, F. Overcoming barriers in Pseudomonas aeruginosa lung infections: Engineered nanoparticles for local delivery of a cationic antimicrobial peptide. Colloids Surf. B Biointerfaces 2015, 135, 717–725.
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in anti-biofilm efficacy of lipid–polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2011, 389, 158–165.
- Gomez-Sotomayor, R.; Ahualli, S.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Iron/Magnetite Nanoparticles as Magnetic Delivery Systems for Antitumor Drugs. J. Nanosci. Nanotechnol. 2015, 15, 3507–3514.
- Reyes-Ortega, F.; Delgado, A.V.; Schneider, E.K.; Checa Fernandez, B.L.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymers 2017, 10, 10.
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305.
- Moreno, R.; Poyser, S.; Meilak, D.; Meo, A.; Jenkins, S.; Lazarov, V.K.; Vallejo-Fernandez, G.; Majetich, S.; Evans, R.F.L. The role of faceting and elongation on the magnetic anisotropy of magnetite Fe3O4 nanocrystals. Sci. Rep. 2020, 10, 2722.
- Li, J.; Nickel, R.; Wu, J.; Lin, F.; van Lierop, J.; Liu, S. A new tool to attack biofilms: Driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 2019, 11, 6905–6915.
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When nanoparticles meet biofilms-interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 2015, 6, 591.
- Schneider, E.K.; Reyes-Ortega, F.; Li, J.; Velkov, T. Can Cystic Fibrosis Patients Finally Catch a Breath With Lumacaftor/Ivacaftor? Clin. Pharm. Ther. 2017, 101, 130–141.
- Döring, G.; Flume, P.; Heijerman, H.; Elborn, J.S. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012, 11, 461–479.
- Schneider, E.K.; Azad, M.A.; Han, M.L.; Tony Zhou, Q.; Wang, J.; Huang, J.X.; Cooper, M.A.; Doi, Y.; Baker, M.A.; Bergen, P.J.; et al. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2016, 2, 478–488.
- Armijo, L.; Kopciuch, M.; Olszόwka, Z.; Wawrzyniec, S.; Rivera, A.; Plumley, J.; Cook, N.; Brandt, Y.; Huber, D.; Smolyakov, G.; et al. Delivery of Tobramycin Coupled to Iron Oxide Nanoparticles across the Biofilm of Mucoidal Pseudonomas Aeruginosa and Investigation of Its Efficacy; SPIE: Bellingham, WA, USA, 2014; Volume 8955.
- Velino, C.; Carella, F.; Adamiano, A.; Sanguinetti, M.; Vitali, A.; Catalucci, D.; Bugli, F.; Iafisco, M. Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis. Front. Bioeng. Biotechnol. 2019, 7, 406.
- Koerner-Rettberg, C.; Ballmann, M. Colistimethate sodium for the treatment of chronic pulmonary infection in cystic fibrosis: An evidence-based review of its place in therapy. Core Evid. 2014, 9, 99–112.
- Tan, M.; Reyes-Ortega, F.; Schneider, E. Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry 2020, 6, 25.
- Yeh, Y.-C.; Huang, T.-H.; Yang, S.-C.; Chen, C.-C.; Fang, J.-Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 8.
- El-Sherbiny, I.M.; Elbaz, N.M.; Sedki, M.; Elgammal, A.; Yacoub, M.H. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine (Lond.) 2017, 12, 387–402.
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.-P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of Coated Silver Nanoparticles to Understand the Relationship of Particle Dissolution and Bioavailability to Cell and Lung Toxicological Potential. Small 2014, 10, 385–398.
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Morrell-Falvey, J.L.; Gu, B.; Doktycz, M.J. Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir 2012, 28, 2727–2735.
- Gauger, A.J.; Hershberger, K.K.; Bronstein, L.M. Theranostics Based on Magnetic Nanoparticles and Polymers: Intelligent Design for Efficient Diagnostics and Therapy. Front. Chem. 2020, 8, 561.
- Santos-Martinez, M.J.; Rahme, K.; Corbalan, J.J.; Faulkner, C.; Holmes, J.D.; Tajber, L.; Medina, C.; Radomski, M.W. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1004–1015.
- Guo, L.-M.; Xu, X.-M.; Zhao, D.; Cai, X.-G.; Zhou, B. Biosynthesis, characterization of PLGA coated folate-mediated multiple drug loaded copper oxide (CuO) nanoparticles and it’s cytotoxicity on nasopharyngeal cancer cell lines. AMB Express 2020, 10, 160.
- Hedayatnasab, Z.; Dabbagh, A.; Abnisa, F.; Wan Daud, W.M.A. Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur. Polym. J. 2020, 133, 109789.
- Yin, J.-J.; Wahid, F.; Zhang, Q.; Tao, Y.-C.; Zhong, C.; Chu, L.-Q. Facile Incorporation of Silver Nanoparticles into Quaternized Poly(2-(Dimethylamino)Ethyl Methacrylate) Brushes as Bifunctional Antibacterial Coatings. Macromol. Mater. Eng. 2017, 302, 1700069.
- Kurzhals, S.; Zirbs, R.; Reimhult, E. Synthesis and Magneto-Thermal Actuation of Iron Oxide Core–PNIPAM Shell Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19342–19352.
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and pH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971.
- Palacios-Ponce, S.; Ramos-González, R.; Ruiz, H.A.; Aguilar, M.A.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; Aguilar, C.N.; Michelena, G.; Ilyina, A. Trichoderma sp. spores and Kluyveromyces marxianus cells magnetic separation: Immobilization on chitosan-coated magnetic nanoparticles. Prep. Biochem. Biotechnol. 2017, 47, 554–561.
- Savelyev, Y.; Gonchar, A.; Movchan, B.; Gornostay, A.; Vozianov, S.; Rudenko, A.; Rozhnova, R.; Travinskaya, T. Antibacterial polyurethane materials with silver and copper nanoparticles. Mater. Today Proc. 2017, 4, 87–94.
- Wang, R.; Degirmenci, V.; Xin, H.; Li, Y.; Wang, L.; Chen, J.; Hu, X.; Zhang, D. PEI-Coated Fe3O4 Nanoparticles Enable Efficient Delivery of Therapeutic siRNA Targeting REST into Glioblastoma Cells. Int. J. Mol. Sci. 2018, 19, 2230.
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890.
- Abedin, M.R.; Umapathi, S.; Mahendrakar, H.; Laemthong, T.; Coleman, H.; Muchangi, D.; Santra, S.; Nath, M.; Barua, S. Polymer coated gold-ferric oxide superparamagnetic nanoparticles for theranostic applications. J. Nanobiotechnol. 2018, 16, 80.
- Korani, M.; Ghazizadeh, E.; Korani, S.; Hami, Z.; Mohammadi-Bardbori, A. Effects of silver nanoparticles on human health. Eur. J. Nanomed. 2015, 7, 51–62.
- Munger, M.A.; Radwanski, P.; Hadlock, G.C.; Stoddard, G.; Shaaban, A.; Falconer, J.; Grainger, D.W.; Deering-Rice, C.E. In vivo human time-exposure study of orally dosed commercial silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1–9.
- Balfourier, A.; Luciani, N.; Wang, G.; Lelong, G.; Ersen, O.; Khelfa, A.; Alloyeau, D.; Gazeau, F.; Carn, F. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. USA 2020, 117, 103.
- Talapko, J.; Matijević, T.; Juzbašić, M.; Antolović-Požgain, A.; Škrlec, I. Antibacterial Activity of Silver and Its Application in Dentistry, Cardiology and Dermatology. Microorganisms 2020, 8, 1400.
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67.
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763.
- Dobson, J. Magnetic Micro- and Nano-particle-based Targeting for Drug and Gene Delivery. Nanomedicine 2006, 1, 31–37.
- Hanuš, J.; Ullrich, M.; Dohnal, J.; Singh, M.; Stěpánek, F. Remotely controlled diffusion from magnetic liposome microgels. Langmuir 2013, 29, 4381–4387.
- Dong, D.; Thomas, N.; Thierry, B.; Vreugde, S.; Prestidge, C.A.; Wormald, P.-J. Distribution and Inhibition of Liposomes on Staphylococcus aureus and Pseudomonas aeruginosa Biofilm. PLoS ONE 2015, 10, e0131806.
- Anupama, S.; Rishabha, M.; Pramod, K.S. Pulmonary Drug Delivery System: A Novel Approach for Drug Delivery. Curr. Drug Ther. 2011, 6, 137–151.
- Joshi, M.; Nisra, A. Pulmonary disposition of budesonide from liposomal dry powder inhaler. Methods Find Exp. Clin. Pharmacol. 2001, 23, 531.
- Meers, P.; Neville, M.; Malinin, V.; Scotto, A.W.; Sardaryan, G.; Kurumunda, R.; Mackinson, C.; James, G.; Fisher, S.; Perkins, W.R. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J. Antimicrob. Chemother. 2008, 61, 859–868.
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244.
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155.
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700.
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcon Godoy, C.; Cayupe Rivas, B.; Gonzalez-Casanova, J.; Rojas-Gomez, D.; Caro Fuentes, N. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551.
- Shi, S.F.; Jia, J.F.; Guo, X.K.; Zhao, Y.P.; Chen, D.S.; Guo, Y.Y.; Zhang, X.L. Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 6499–6506.
- Subbiahdoss, G.; Sharifi, S.; Grijpma, D.W.; Laurent, S.; van der Mei, H.C.; Mahmoudi, M.; Busscher, H.J. Magnetic targeting of surface-modified superparamagnetic iron oxide nanoparticles yields antibacterial efficacy against biofilms of gentamicin-resistant staphylococci. Acta Biomater. 2012, 8, 2047–2055.
- Wang, X.; Deng, A.; Cao, W.; Li, Q.; Wang, L.; Zhou, J.; Hu, B.; Xing, X. Synthesis of chitosan/poly(ethylene glycol)-modified magnetic nanoparticles for antibiotic delivery and their enhanced anti-biofilm activity in the presence of magnetic field. J. Mater. Sci. 2018, 53, 6433–6449.
- Tange, R.A.; Dreschler, W.A.; Prins, J.M.; Buller, H.R.; Kuijper, E.J.; Speelman, P. Ototoxicity and nephrotoxicity of gentamicin vs netilmicin in patients with serious infections. A randomized clinical trial. Clin. Otolaryngol. Allied Sci. 1995, 20, 118–123.
- Quan, K.; Zhang, Z.; Ren, Y.; Busscher, H.J.; van der Mei, H.C.; Peterson, B.W. Homogeneous Distribution of Magnetic, Antimicrobial-Carrying Nanoparticles through an Infectious Biofilm Enhances Biofilm-Killing Efficacy. ACS Biomater. Sci. Eng. 2020, 6, 205–212.
- Chen, T.; Wang, R.; Xu, L.Q.; Neoh, K.G.; Kang, E.-T. Carboxymethyl Chitosan-Functionalized Magnetic Nanoparticles for Disruption of Biofilms of Staphylococcus aureus and Escherichia coli. Ind. Eng. Chem. Res. 2012, 51, 13164–13172.
- Günday Türeli, N.; Torge, A.; Juntke, J.; Schwarz, B.C.; Schneider-Daum, N.; Türeli, A.E.; Lehr, C.M.; Schneider, M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 2017, 117, 363–371.
- Thomas, N.; Thorn, C.; Richter, K.; Thierry, B.; Prestidge, C. Efficacy of Poly-Lactic-Co-Glycolic Acid Micro- and Nanoparticles of Ciprofloxacin Against Bacterial Biofilms. J. Pharm. Sci. 2016, 105, 3115–3122.
- Ernst, J.; Klinger-Strobel, M.; Arnold, K.; Thamm, J.; Hartung, A.; Pletz, M.W.; Makarewicz, O.; Fischer, D. Polyester-based particles to overcome the obstacles of mucus and biofilms in the lung for tobramycin application under static and dynamic fluidic conditions. Eur. J. Pharm. Biopharm. 2018, 131, 120–129.
- Hua, X.; Tan, S.; Bandara, H.M.H.N.; Fu, Y.; Liu, S.; Smyth, H.D.C. Externally Controlled Triggered-Release of Drug from PLGA Micro and Nanoparticles. PLoS ONE 2014, 9, e114271.
- Mariela, A.; Agotegaray, V.L.L. Silica-Coated Magnetic Nanoparticles: An Insight into Targeted Drug Delivery and Toxicology; Springer: Cham, Switzerland, 2017.
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789.
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329.




