| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Huarong Chen | + 4582 word(s) | 4582 | 2020-12-14 07:52:13 | | | |
| 2 | Rita Xu | -2162 word(s) | 2420 | 2020-12-21 08:02:48 | | | | |
| 3 | Rita Xu | + 53 word(s) | 2473 | 2020-12-22 08:57:02 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and one of the leading causes of cancer-related death worldwide. HCC is highly heterogeneous, both within the tumor and among individuals, which is closely related to the HCC surveillance, diagnosis, prognosis, and treatment response. With the advances of next-generation sequencing, the genomic landscape of HCC has been identified which vastly improves our understanding of genetic and epigenetic changes and their interaction during HCC development.
1. Introduction
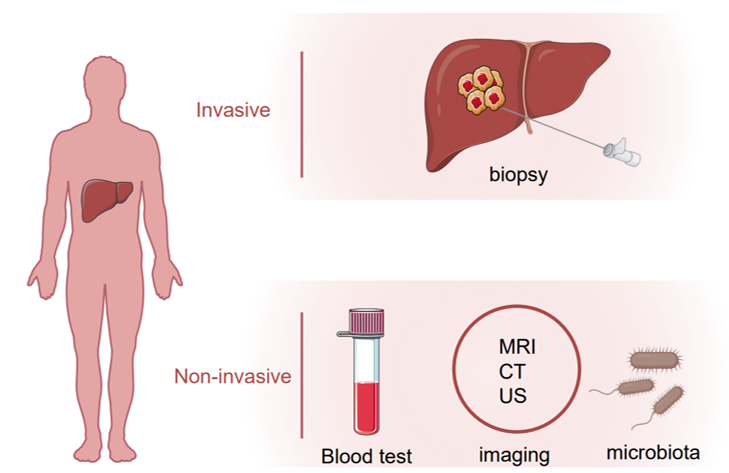
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the fourth-leading cause of cancer death worldwide [1]. Several risk factors are known to contribute to HCC development including the hepatitis B or C virus (HBV or HCV) infection, alcohol abuse, obesity and non-alcoholic fatty liver disease (NAFLD) [2]. For HCC patients at an early stage, surgical resection or percutaneous ablation is recommended as a first-line treatment option, and the recurrence rate five years after surgery is around 50% [3][4][5]. On the other hand, more than half of HCC patients were diagnosed with advanced or unresectable disease with a very poor prognosis due to extremely limited therapeutic options [6]. Early detection of HCC using imaging and tumor markers could dramatically improve patient outcomes. For patients with cirrhosis, surveillance of HCC is recommended which endorses significant benefits [7]. Several biomarkers in body fluid samples, e.g., plasma, serum, urine or stool, have been uncovered that could be objectively measured for HCC surveillance and diagnosis. Alpha-fetoprotein (AFP), AFP-L3 and des-γ-carboxy prothrombin (DCP) are the most well-studied and widely used non-invasive biomarkers in HCC. Apart from them, many other molecules such as Glypican 3 (GPC-3), Alpha-l fucosidase (AFU), Golgi protein-73 (GP73) and Squamous cell carcinoma antigen (SCCA), or tumor-associated signatures such as DNA mutation, DNA methylation, micro-RNAs (miRNAs) and long non-coding RNAs (lncRNAs) are under investigation that could be taken into consideration for future clinical practice. In this review, we summarize current HCC biomarker studies, highlighting novel biomarkers and imaging tests that may improve surveillance and diagnosis of HCC in the future (Figure 1).
Figure 1. Invasive and noninvasive assessment of hepatocellular carcinoma (HCC).
2. Etiologic Factors of HCC
Etiologic agents, e.g., HBV, HCV, alcohol and NAFLD, could lead to chronic liver injury or liver cirrhosis and ultimately HCC, and thus are regarded as the risk factors for HCC. The risk factors of HCC vary in different geographic areas. In China, HBV is accountable for around 54% of HCC cases while 31% of liver cancer cases in Egypt are attributed to HCV infection [8]. As for western countries, NAFLD has emerged as an important cause of HCC in recent years [9]. The development of HCC in NAFLD patients may be associated with excessive body weight, hepatic iron-overload and insulin resistance which could contribute to advanced fibrosis and cirrhosis. Asides from these, gender and age are also thought to be risk factors of HCC. Intriguingly, the prevalence of HCC in males is two to four times more common than in females, a situation called gender disparity [10]. The reasons are complex and could be partially explained by the opposite effects of androgens and estrogen. Estradiol, an estrogen steroid hormone, has been reported to upregulate p53 expression thus suppress HCC [11]. On the other hand, testosterone, the predominant androgen, could promote the hepatocyte cell cycle via cyclin E [11]. In combination with other factors such as HBV and/or HCV infections, age is considered as another risk factor of HCC. A multi-center study across six South American countries involving 1336 patients revealed that nearly 40% of HCC patients with HBV infection at diagnosis were before age 50, while most cases with HCV infection were over the age of 60 [12]. Other environmental factors, such as dietary habits, alcohol consumption and exposure to aflatoxin, are also associated with HCC development.
3. Approaches to Identify Potential HCC Biomarkers
Biomarkers are defined as measurable indicators of physiological or pathological processes, or in response to various diagnostic or therapeutic procedures. The development of HCC is characterized by multiple genetic and epigenetic events alterations that run through cancer initiation, promotion and progression. During this process, liver cells are likely to present different molecular signatures, and release certain tumor-associated molecules into body fluid, e.g., blood, urine or stool, that could be monitored for the onset or progression of HCC. The development of detection technology has vastly advanced the development of HCC biomarkers. At present, many biotechnologies have been applied, such as chemiluminescence immunoassay, enzyme-linked immunosorbent assay, immunosensor, proteomics, liquid biopsy, and so on. The advent of Next-Generation Sequencing (NGS) has significantly increased our ability to look into the molecular pathogenesis and heterogeneity of HCC as well as a range of HCC biomarkers, including gene mutations, epigenetic modifications, aberrant expression of coding and non-coding RNAs, and gut microbiome [13][14][15][16][17][18]. An accurate landscape of HCC genetic and epigenetic alterations has been built up with high-throughput analyses of different cohorts which unravels potential biomarkers for monitoring the HCC imitation and progression [15][17]. Meanwhile, a recent study applied genome-wide 5-hydroxymethylcytosines detection using circulating cell-free DNA samples, providing a non-invasive tool in the early detection of HCC [19]. Another new technology, proteome, measuring global protein abundance and post-translational modifications, provides additional biological insights in HCC [14][20]. This method reveals a multi-omics profile of key signaling and metabolic pathways in HCC [20]. The proteomic profiles of tumor-derived extracellular vesicles and particles in human tissues and blood have been well characterized and can serve as reliable biomarkers [21]. Identification of novel non-invasive biomarkers with reliable analytical techniques will shed light on early diagnosis and management of HCC. In the following sections, we will discuss several HCC biomarkers.
4. Biomarkers for HCC
4.1. Protein Biomarkers
4.1.1. AFP and AFP-L3
Alpha-fetoprotein (AFP) is the most well-studied and commonly used biomarker for the diagnosis and prognosis of HCC [22][23]. AFP is primarily produced by the fetus’s liver and its expression declines rapidly to very a low level by the age of one. However, liver damage or liver cancer can dramatically increase AFP levels in the blood. In a nested case-control study, elevated AFP level could be observed 6 months before the diagnosis of HCC [24], implying that detection of AFP is useful for HCC diagnosis. Current criticisms on the use of AFP mainly focus on its insufficient sensitivity and specificity for early HCC detection if used alone. In addition, increased AFP levels can be found in the setting of cirrhosis patients with active hepatitis, elevated serum alanine aminotransferase (ALT), or non-HCC malignancies [25][26]. To date, AFP detection alone is not recommended for HCC screening. The European Association for the Study of the Liver recommends using liver ultrasound for the surveillance of HCC rather than AFP detection [27]. Nevertheless, the use of AFP is an effective auxiliary diagnostic tool for the detection and surveillance of HCC. In a meta-analysis study comparing the efficacy of ultrasound with or without AFP for early HCC detection (n = 2770), Kristina Tzartzeva et al. showed that the use of AFP in combination with abdominal ultrasound can significantly increases the sensitivity of early HCC detection as compared to ultrasound alone (63% vs. 45%) [28]. Moreover, AFP could be used for monitoring HCC progression considering that it promotes tumor proliferation and metastasis [29][30][31]. A meta-analysis consisting of 29 studies and 4726 HCC patients highlighted that AFP level was a potential noninvasive prognosis marker for HCC patients, and AFP Slope > 7.5 ng/mL per month was associated with HCC recurrence post-liver transplantation [32].
AFP-L3, an isoform of AFP, is specific to malignant tumors. The presence of AFP-L3 can serve to identify patients with a high risk of HCC who require increased monitoring. AFP-L3 has been approved by the US Food and Drug Administration (FDA) for assessing the risk of liver cancer. With a cutoff of 1.7%, the use of AFP-L3 demonstrates a better specificity but lower sensitivity for early HCC detection as compared to AFP [33]. Consistently, in a retrospective study recruiting 104 HCC patients with 104 matched non-HCC individuals, the elevation of AFP-L3 was present before the tumor became visible by imaging even though very low AFP levels could be detected, suggesting that AFP-L3 may serve as an early predictive HCC marker [34]. In the future, whether a combination of AFP-L3 and AFP could achieve better diagnostic efficacy for HCC warrants large population-based cohort studies.
4.1.2. DCP
Des-gamma-carboxy prothrombin (DCP), also known as the protein induced by vitamin K absence or antagonist II (PIVKA-II), is a nonfunctional prothrombin [35]. DCP was described as both an autologous growth factor that promotes HCC growth, and a paracrine factor that participates in the crosstalk between HCC and vascular endothelial cells. The biological malignant potential of DCP and its abnormal expression in HCC tissues pinpoints its potential for HCC prediction. In a study involving 1377 HCC patients and 355 patients with chronic hepatitis or cirrhosis, Shinichiro Nakamura et al. compared the diagnostic efficacy of DCP and AFP in discriminating HCC from chronic liver diseases [36]. The results demonstrated that DCP was superior to AFP in detecting large tumors (greater than 5 cm in diameter) [36]. In addition, DCP is a potential prognostic factor for patients with HCC after treatment. In a single-centre retrospective study comprising 412 patients with HBV-related HCC who were treated with radiofrequency ablation, DCP, but not AFP, was found to be an independent prognostic factor for both recurrence-free and overall survival in these patients [37]. The FDA has approved DCP for use in predicting liver cancer. Notably, DCP, AFP and AFP-L3 have been recommended for clinical practice according to Chinese and Japanese guideline [38][39]. However, a recent study carried out in Korea showed that a combination of DCP, AFP and AFP-L3 did not improve the performance for early HCC detection as compared to either AFP or AFP-L3 alone [24]. Further studies are required to evaluate the contribution of DCP for early HCC detection.
4.1.3. GPC-3
Glypican 3 (GPC-3) is a heparan sulfate proteoglycan that plays an important role in cell proliferation and differentiation, and is found to be highly associated with tumor development [40].
GPC-3 has emerged as a potential target for the diagnosis and treatment of HCC recently. GPC3 is rarely expressed in normal hepatocytes or pathological liver cells of hepatitis and cirrhosis. In contrast, GPC-3 is specifically overexpressed in HCC tissues [41]. Consistent results were reported that both GPC-3 mRNA and protein expressions were upregulated in HCC tissues [40][42]. However, the detection of GPC-3 in blood was not as effective as that in tissue biopsies for the diagnosis of HCC [43][44]. By examining serum GPC3 levels in HCC patients using enzyme-linked immunosorbent assay (ELISA), 36.1% to 95% of positive cases could be identified as reported by different studies [45]. Furthermore, serum GPC3 levels were comparable between patients without HCC and those with early HCC [45]. Additional investigations should be carried out to assess the potential of serum GPC3 as non-invasive diagnostic marker for HCC.
4.1.4. AFU
Alpha-l fucosidase (AFU) is a lysosomal enzyme and is reported to participate in the degradation of various fucose-containing fucoglycoconjugates. AFU has been proposed as a potential tumor marker in the diagnosis of HCC. At the cut-off value of 24 U/I, the area under the receiver operating characteristic curve (AUROC) for AFU was 0.83, with sensitivity and specificity of 56.1% and 69.2%, respectively [46]. The diagnostic efficiency of AFU was lower than AFP (cut-off value of 20 ng/mL for AFP) in this study [46]. In contrast, another study involving 1053 HCC patients showed that AFU exerted the same diagnostic power as AFP in both sensitivities (73.52% for AFU vs. 75.01% for AFP) and specificities (76.81% for AFU vs. 82.08% for AFP) [47]. It is worth noting that overexpression of AFP was also observed in other non-HCC diseases such as esophageal squamous cell carcinomas [48] and preeclampsia [49] which could markedly reduce the specificity of AFU for HCC diagnosis.
4.1.5. Other Protein Biomarkers
Proteins that are highly expressed in HCC compared with normal tissues could be promising candidates for HCC detection (Table 1). Golgi protein-73 (GP73, also called Golph2) is a transmembrane glycoprotein primarily expressed in epithelial cells. GP73 has been found upregulated in patients with diverse liver diseases, especially in HCC. In a large cohort study involving more than 4200 serum samples derived from healthy individuals and patients with benign or malignant liver disease, the sensitivity (74.6% for GP73 vs. 58.25% for AFP) and specificity (97.4% for GP73 vs. 85.3% for AFP) of GP73 for detection of HCC were higher than AFP [50]. A consistent result was observed in another study that GP73 had higher diagnostic performance than AFP [51]. Notably, a combination of these two markers could increase the sensitivity for HCC detection to 89.2%, with the specificity of 85.2% [50]. GP73 may also serve as an indicator for the recurrence of HCC given that serum GP73 levels diminished after surgical resection of HCC and rebound after tumor reappeared [50]. Furthermore, serum GP73 level was positively correlated with serum HBV DNA copies and the Child–Pugh score in cirrhotic patients [51]. GP73 is of importance for monitoring the patients with HBV infection who may eventually develop cirrhosis and HCC [51]. GP73 could be used for prediction of HCC in a cirrhotic population. However, in another study, the level of GP73 did not differ among patients with different types of liver disease [52]. Further large-scale and multi-centered studies are needed to evaluate the diagnostic accuracy and surveillance potential of GP73.
Table 1. Biomarkers for HCC diagnosis.
|
Biomarker |
Samples |
Type of Cohort |
Sample Size |
AUROC or Positive Rate (%) |
Sensitivity (%) (95%CI) |
Specificity (%) (95%CI) |
Cutoff Value |
Limitation |
Refs |
|
AFP |
serum |
prospective |
689 |
0.77 |
62 (48–76) |
87 (82–92) |
5 ng/mL |
Modest accuracy |
[24] |
|
AFP-L3 |
serum |
prospective |
689 |
0.73 |
55 (40–69) |
90 (85–94) |
4.0% |
Modest accuracy |
[24] |
|
GPC-3 |
tissue |
|
|
|
|
|
|
Variation among tests |
|
|
|
mRNA |
retrospective |
52–105 |
55.7–100 |
NA |
NA |
NA |
[45] |
|
|
protein |
|
107–757 |
63.6–91 |
NA |
NA |
NA |
[51] |
||
|
GPC-3 |
serum |
retrospective |
60–625 |
31.6–95 |
NA |
NA |
|
||
|
DCP |
serum |
retrospective |
689 |
0.71 |
48 (33–64) |
86 (80–91) |
NA |
Modest accuracy |
[24] |
|
AFU |
serum |
retrospective |
512 |
0.68 |
56.1 (NA) |
69.2 (NA) |
24 U/I |
Low accuracy |
[46] |
|
GP73 |
serum |
retrospective |
60–4217 |
0.73–0.94 |
72.4–74.6 |
61.5–97.4 |
different cutoff |
Modest accuracy |
|
|
SCCA |
serum |
meta-analysis |
12 studies |
0.53–0.9 |
12–84 |
48–100 |
different cutoff |
Low accuracy |
|
|
SCCA-IgM |
serum |
meta-analysis |
12 studies |
0.66–0.86 |
51–89 |
48–78 |
different cutoff |
Low accuracy |
NA: not available.
Squamous cell carcinoma antigen (SCCA) is composed of two highly homologous proteins SCCA1 and SCCA2 and belongs to the serine protease inhibitor family. SCCA is reported to participate in multiple biological processes such as cell proliferation, resistance to apoptosis, and epithelial–mesenchymal transition. Overexpression of SSCA was identified in HCC tissues at an early stage, indicating that it could be a potential candidate for HCC diagnosis [56]. Following studies were carried out to evaluate the diagnostic value of SCCA [53][55], showing that SCCA complexed with IgM (SCCA-IgM) was useful for assessment of HCC in cirrhotic patients with high sensitivity but poor specificity. A meta-analysis involving 11 studies concluded that both SCCA and SCCA-IgM presented diagnostic value for HCC, with AUROC of 0.8 and 0.77, respectively [57]. Moreover, SCCA can be used to predict the prognosis of HCC patients, thus is recommended to be included in clinical practice in some studies [53].
Others candidates include Apelin [58], β2 microglobulin [59], dickkopf-1 [60], GATA Zinc Finger Domain Containing 1 [61], osteopontin [62] and squalene epoxidase [63] that are reported to have abnormal expressions in HCC as compared to normal control. Although these markers are reported as sensitive biomarkers for HCC prediction, they have not yet been applied in clinical or recommended for use by major professional hepatology societies, probably because of limited sample size, lack of external validation, or sample accessibility, implying the complexity and challenges of biomarker development. Large prospective studies are needed to further validate their performance in HCC diagnosis and prognosis.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin Nov, 2018, 68, 394–424.
- Villanueva, A. Hepatocellular Carcinoma. Engl. J. Med. 2019, 380, 1450–1462.
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Huang, Y.H.; Chiou, Y.Y.; Lin, H.C.; Huo, T.I. Surgical Resection Versus Radiofrequency Ablation for Single Hepatocellular Carcinoma
- Kao, W.Y.; Chao, Y.; Chang, C.C.; Li, C.P.; Su, C.W.; Huo, T.I.; Huang, Y.H.; Chang, Y.J.; Lin, H.C.; Wu, J.C. Prognosis of Early-Stage Hepatocellular Carcinoma: The Clinical Implications of Substages of Barcelona Clinic Liver Cancer System Based on a Cohort of 1265 Patients. Medicine 2015, 94, e1929.
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated with Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120.
- Colagrande, S.; Inghilesi, A.L.; Aburas, S.; Taliani, G.G.; Nardi, C.; Marra, F. Challenges of advanced hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7645–7659.
- Yang, J.D.; Mannalithara, A.; Piscitello, A.J.; Kisiel, J.B.; Gores, G.J.; Roberts, L.R.; Kim, W.R. Impact of surveillance for hepatocellular carcinoma on survival in patients with compensated cirrhosis. Hepatology 2018, 68, 78–88.
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular carcinoma. Rev. Dis. Primers 2016, 2, 16018.
- Seydel, G.S.; Kucukoglu, O.; Altinbasv, A.; Demir, O.O.; Yilmaz, S.; Akkız, H.; Otan, E.; Sowa, J.-P.; Canbay, A. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Hepatol. 2016, 15, 662–672.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. J. Cancer 2015, 136, E359–E386.
- Pok, S.; Wong, H.J.; Board, P.; Barn, V.A.; Blackburn, A.C.; Farrell, G.C.; Teoh, N.C. Testosterone regulation of cyclin E kinase: A key factor in determining gender differences in hepatocarcinogenesis. Gastroenterol. Hepatol. 2016, 31, 1210–1219, doi:10.1111/jgh.13232.
- Chan, A.J.; Balderramo, D.; Kikuchi, L.; Ballerga, E.G.; Prieto, J.E.; Tapias, M.; Idrovo, V.; Davalos, M.B.; Cairo, F.; Barreyro, F.J.; et al. Early Age Hepatocellular Carcinoma Associated with Hepatitis B Infection in South America. Gastroenterol. Hepatol. 2017, 15, 1631–1632, doi:10.1016/j.cgh.2017.05.015.
- Wu, Z.-H.; Yang, D.-L. Identification of a protein signature for predicting overall survival of hepatocellular carcinoma: A study based on data mining. BMC Cancer 2020, 20, 1–9.
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Cell Biol. 2019, 567, 257–261.
- Schulze, K.; Nault, J.-C.; Villanueva, A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. Hepatol. 2016, 65, 1031–1042.
- Duan, J.; Wu, Y.; Liu, J.; Zhang, J.; Fu, Z.; Feng, T.; Liu, M.; Han, J.; Li, Z.; Chen, S. Genetic Biomarkers for Hepatocellular Carcinoma in the Era of Precision Medicine. Hepatocell. Carcinoma 2019, 6, 151–166.
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.-C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4.
- Huang, H.; Ren, Z.; Gao, X.; Hu, X.; Zhou, Y.; Jiang, J.; Lu, H.; Yin, S.; Ji, J.; Zhou, L.; et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020, 12, 1–14.
- Cai, J.; Chen, L.; Zhang, Z.; Zhang, X.; Lu, X.; Liu, W.; Shi, G.; Ge, Y.; Gao, P.; Yang, Y.; et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut 2019, 68, 2195–2205.
- Gao, Q.; Zhu, H.; Dong, L.; Shi, W.; Chen, R.; Song, Z.; Huang, C.; Li, J.; Dong, X.; Zhou, Y.; et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell 2019, 179, 561–577.e22.
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18.
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Int. 2017, 11, 317–370.
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750.
- Choi, J.; Kim, G.; Han, S.; Lee, W.; Chun, S.; Lim, Y. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early‐Stage Hepatocellular Carcinoma. Hepatology 2019, 69, 1983–1994.
- Yang, J.D.; Dai, J.; Singal, A.G.; Gopal, P.; Addissie, B.D.; Nguyen, M.H.; Befeler, A.S.; Reddy, K.R.; Schwartz, M.; Harnois, D.M.; et al. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1085–1092.
- Di Bisceglie, A.M.; Sterling, R.K.; Chung, R.T.; Everhart, J.E.; Dienstag, J.L.; Bonkovsky, H.L.; Wright, E.C.; Everson, G.T.; Lindsay, K.L.; Lok, A.S.; et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: Results from the HALT-C Trial. Hepatol. 2005, 43, 434–441.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018, 69, 182–236.
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1.
- Li, M.-S.; Li, P.-F.; He, S.-P.; Du, G.-G.; Li, G. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J. Gastroenterol. 2002, 8, 469–475.
- Li, M.; Li, P.-F.; Chen, Q.; Du, G.-G.; Li, G. Alpha-fetoprotein stimulated the expression of some oncogenes in human hepatocellular carcinoma Bel 7402 cells. World J. Gastroenterol. 2004, 10, 819–824.
- Lu, Y.; Zhu, M.; Li, W.; Lin, B.; Dong, X.; Chen, Y.; Xie, X.; Guo, J.; Li, M. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. Cell. Mol. Med. 2016, 20, 549–558.
- Giard, J.-M.; Mehta, N.; Dodge, J.L.; Roberts, J.P.; Yao, F.Y. α-Fetoprotein Slope >7.5 ng/mL per Month Predicts Microvascular Invasion and Tumor Recurrence after Liver Transplantation for Hepatocellular Carcinoma. Transplantation 2018, 102, 816–822.
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. α-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118.
- Kumada, T.; Toyoda, H.; Tada, T.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Tanaka, J.; Kagebayashi, C.; Satomura, S. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. Gastroenterol. 2014, 49, 555–563.
- Lim, T.S.; Rhee, H.; Kim, G.M.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Ahn, S.H.; Han, K.-H.; Choi, J.-Y.; Kim, D.Y. Alpha-Fetoprotein, Des-Gamma-Carboxy Prothrombin, and Modified RECIST Response as Predictors of Survival after Transarterial Radioembolization for Hepatocellular Carcinoma. Vasc. Interv. Radiol. 2019, 30, 1194–1200.e1.
- Nakamura, S.; Nouso, K.; Sakaguchi, K.; Ito, Y.M.; Ohashi, Y.; Kobayashi, Y.; Toshikuni, N.; Tanaka, H.; Miyake, Y.; Matsumoto, E.; et al. Sensitivity and Specificity of Des-Gamma-Carboxy Prothrombin for Diagnosis of Patients with Hepatocellular Carcinomas Varies According to Tumor Size. J. Gastroenterol. 2006, 101, 2038–2043.
- Lee, S.; Rhim, H.; Kim, Y.-S.; Kang, T.; Song, K.D. Post-ablation des-gamma-carboxy prothrombin level predicts prognosis in hepatitis B-related hepatocellular carcinoma. Liver Int. 2015, 36, 580–587, doi:10.1111/liv.12991.
- Kokudo, N.; Hasegawa, K.; Akahane, M.; Igaki, H.; Izumi, N.; Ichida, T.; Uemoto, S.; Kaneko, S.; Kawasaki, S.; Ku, Y.; et al. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Res. 2015, 45, doi:10.1111/hepr.12464.
- Xie, D.-Y.; Ren, Z.-G.; Zhou, J.; Fan, J.; Gao, Q. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. HepatoBiliary Surg. Nutr. 2017, 6, 387–396, doi:10.21037/hbsn.2017.11.01.
- Capurro, M.; Wanless, I.R.; Sherman, M.; DeBoer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97.
- Hsu, H.C.; Cheng, W.; Lai, P.L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: Biological significance and temporospatial distribution. Cancer Res. 1997, 57, 5179–5184.
- Zhu, Z.-W.; Friess, H.; Wang, L.; Abou-Shady, M.; Zimmermann, A.; Lander, A.D.; Korc, M.; Kleeff, J.; Büchler, M.W. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001, 48, 558–564.
- Nault, J.-C.; Guyot, E.; Laguillier, C.; Chevret, S.; Ganne-Carrie, N.; N’Kontchou, G.; Beaugrand, M.; Seror, O.; Trinchet, J.-C.; Coelho, J.; et al. Serum Proteoglycans as Prognostic Biomarkers of Hepatocellular Carcinoma in Patients with Alcoholic Cirrhosis. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1343–1352.
- Shafizadeh, N.; Ferrell, L.D.; Kakar, S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Pathol. 2008, 21, 1011–1018.
- Zhou, F.; Shang, W.; Yu, X.; Tian, J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Res. Rev. 2018, 38, 741–767.
- Xing, H.; Qiu, H.; Ding, X.; Han, J.; Li, Z.; Wu, H.; Yan, C.; Li, H.; Han, R.; Zhang, H.; et al. Clinical performance of α-L-fucosidase for early detection of hepatocellular carcinoma. Med. 2019, 13, 545–555.
- Xi, L.; Yang, C. Evaluation of alpha-l-fucosidase for the diagnosis of hepatocellular carcinoma based on meta-analysis. Lab. Med. 2020, 44, 183–189.
- Yu, X.; Zhang, R.; Yang, T.; Zhang, M.; Xi, K.; Lin, Y.; Wen, Y.; Wang, G.; Huang, Z.; Zhang, X.; et al. Alpha-l-fucosidase: A novel serum biomarker to predict prognosis in early stage esophageal squamous cell carcinoma. Thorac. Dis. 2019, 11, 3980–3990.
- Zhang, M.; Wang, L.; Zhang, H.; Cong, J.; Zhang, L. Serum α-l-fucosidase activities are significantly increased in patients with preeclampsia. Mol. Biol. Transl. Sci. 2019, 162, 349–362, doi:10.1016/bs.pmbts.2018.12.008.
- Mao, Y.; Yang, H.; Xu, H.; Lu, X.; Sang, X.; Du, S.; Zhao, H.; Chen, W.; Xu, Y.; Chi, T.; et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010, 59, 1687–1693.
- Ali, O.M.; El Amin, H.A.; Sharkawy, Y.L.; Ali, A.A.M.; Kholef, E.F.M.; Elsewify, W.A.E. Golgi Protein 73 versus Alpha-Fetoprotein as a New Biomarker in Early Diagnosis of Hepatocellular Carcinoma. J. Gen. Med. 2020, 13, 193–200.
- Gu, Y.; Chen, W.; Zhao, Y.; Chen, L.; Peng, T. Quantitative analysis of elevated serum Golgi protein-73 expression in patients with liver diseases. Clin. Biochem. Int. J. Lab. Med. 2009, 46 Pt 1, 38–43, doi:10.1258/acb.2008.008088.
- Pozzan, C.; Cardin, R.; Piciocchi, M.; Cazzagon, N.; Maddalo, G.; Vanin, V.; Giacomin, A.; Pontisso, P.; Cillo, U.; Farinati, F. Diagnostic and prognostic role of SCCA-IgM serum levels in hepatocellular carcinoma (HCC). Gastroenterol. Hepatol. 2014, 29, 1637–1644.
- Zhang, J.; Yu, Y.; Li, Y.; Wei, L. Diagnostic value of contrast-enhanced ultrasound in hepatocellular carcinoma: A meta-analysis with evidence from 1998 to 2016. Oncotarget 2017, 8, 75418–75426.
- Beneduce, L.; Castaldi, F.; Marino, M.; Quarta, S.; Ruvoletto, M.; Benvegnù, L.; M.D., F.C.; Gatta, A.; Pontisso, P.; Fassina, G. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer 2005, 103, 2558–2565.
- Pontisso, P.; Calabrese, F.; Benvegnù, L.; Lise, M.; Belluco, C.; Ruvoletto, M.G.; De Falco, S.; Marino, M.; Valente, M.; Nitti, D.; et al. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. J. Cancer 2004, 90, 833–837.
- Yu, J.; Wang, Z.-J.; Chen, L.-H.; Dong, W.-Z. Diagnostic value of serum squamous cell carcinoma antigen for hepatocellular carcinoma: A systematic review and meta-analysis. J. Clin. Lab. Investig. 2016, 77, 8–14.
- Chen, H.; Wong, C.-C.; Liu, D.; Go, M.Y.; Wu, B.; Peng, S.; Kuang, M.; Wong, N.; Yu, J. APLN promotes hepatocellular carcinoma through activating PI3K/Akt pathway and is a druggable target. Theranostics 2019, 9, 5246–5260.
- Okoror, L.E.; Ajayi, A.O.; Ijalana, O.B. Elevated serum beta2-microglobulin in individuals coinfected with hepatitis B and hepatitis D virus in a rural settings in Southwest Nigeria. BMC Res. Notes 2017, 10, 719.
- Zhang, R.; Lin, H.M.; Broering, R.; Yu, X.H.; Xu, L.B.; Wu, W.R.; Liu, C. Dickkopf-1 contributes to hepatocellular carcinoma tumorigenesis by activating the Wnt/beta-catenin signaling pathway. Signal Target. Ther. 2019, 4, 1–10.
- Sun, W.; Zhang, Y.; Wong, K.C.; Liu, K.; Yang, Y.; Wu, B.; Tong, J.H.; Chan, A.W.H.; Chan, H.L.-Y.; Yu, J. Increased expression of GATA zinc finger domain containing 1 through gene amplification promotes liver cancer by directly inducing phosphatase of regenerating liver 3. Hepatology 2018, 67, 2302–2319, doi:10.1002/hep.29750.
- Da Costa, A.N.; Plymoth, A.; Santos‐Silva, D.; Ortiz‐Cuaran, S.; Camey, S.; Guilloreau, P.; Sangrajrang, S.; Khuhaprema, T.; Mendy, M.; Lesi, O.A.; et al. Osteopontin and latent-TGF beta binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. J. Cancer 2015, 136, 172–181.
- Liu, D.; Wong, C.C.; Fu, L.; Chen, H.; Zhao, L.; Li, C.; Zhou, Y.; Zhang, Y.; Xu, W.; Yang, Y.; et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Transl. Med. 2018, 10, eaap9840, doi:10.1126/scitranslmed.aap9840.