
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabella Orienti | + 1410 word(s) | 1410 | 2020-06-04 04:40:27 | | | |
| 2 | Rita Xu | -255 word(s) | 1155 | 2020-06-11 11:11:10 | | | | |
| 3 | Rita Xu | -10 word(s) | 1145 | 2020-10-27 09:36:21 | | | | |
| 4 | Rita Xu | + 371 word(s) | 1516 | 2020-11-09 09:50:13 | | |
Video Upload Options
At present, there is no vaccine or effective standard treatment for SARS-CoV-2 infection (COVID-19) which frequently leads to lethal pulmonary inflammatory responses. COVID-19 pathology is characterized by extreme inflammation and amplified immune response with activation of a cytokine storm. A subsequent progression to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) can take place, which is often followed by death. The causes of these strong inflammatory responses in SARS-CoV-2 infection are still unknown. As uncontrolled pulmonary inflammation is likely the main cause of death in SARS-CoV-2 infection, anti-inflammatory therapeutic interventions are particularly important. Fenretinide N‐(4‐hydroxyphenyl) retinamide is a bioactive molecule characterized by poly-pharmacological properties and a low toxicity profile. Fenretinide is endowed with anti-tumor, anti-inflammatory, anti-viral and immunomodulating properties other than efficacy in obesity/diabetic pathologies. Its anti-inflammatory and anti-viral activities, in particular, could likely have utility in multimodal therapies for treatment of ALI/ARDS in COVID-19 patients. Moreover, fenretinide administration by pulmonary delivery systems could further increase its therapeutic value by carrying high drug concentrations to the lungs and triggering a rapid onset of activity. This is particularly important in SARS-CoV-2 infection where only a narrow time window exists for therapeutic intervention.
1. Introduction
Coronaviruses (CoVs) are RNA viruses. They may infect both humans and animals, leading to lethal and contagious diseases, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). The recent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (or coronavirus disease-19 (COVID-19)) is characterized by high genetic homology with SARS-CoV and MERS-CoV [1], sharing with them 79.0% and 51.8% nucleotide identity, respectively. Several studies have shown that SARS-CoV predominantly infects airway and alveolar epithelial cells, vascular endothelial cells, and macrophages. SARS-CoV-2 and SARS-CoV use the same receptor, angiotensin-converting enzyme 2 (ACE2), for infection, indicating that the same cell types are targeted and infected [2][3].
In SARS-CoV-2 infection, the early onset of a rapid viral replication may cause extensive apoptosis of epithelial and endothelial cells and vascular leakage. This frequently leads to acute lung injury and lethal inflammatory responses [4]. Although antiviral drugs, glucocorticoids, and mechanical ventilation have been used, there is no specific treatment for COVID-19 at the moment [5]. Therefore, in the absence of a standard therapeutic intervention, the urgent treatment of pulmonary inflammation to prevent acute lung injury is needed. Pulmonary formulations of anti-inflammatory drugs could represent a good option in combination with systemic antiviral drugs or glucocorticoids. In pulmonary administration, the drugs are directly carried to the airway and alveolar epithelia in high concentration, providing rapid onset of the therapeutic response with prompt relief of lung occlusion and respiratory distress symptoms. Nevertheless, the drugs used by pulmonary administration in COVID-19 should be characterized by low toxicity to avoid injury to the airway and alveolar epithelia already damaged by the viral infection. Fenretinide—(N-(4-hydroxyphenyl) retinamide)—is a semisynthetic derivative of all-trans-retinoic acid. It is endowed with many pharmacological features, including anti-inflammatory and antiviral activities, prevention of obesity and type-2 diabetes [6], and the well-known antitumor activity on a wide range of tumors [7][8][9][10][11][12][13][14][15][16]. Moreover, its low toxicity profile has been proved in many clinical trials, also in long term treatments [12][17][18]. Therefore, due to its poly-pharmacology, fenretinide administration by pulmonary formulations may be expected to be protective against acute lung injury (ALI)/ acute respiratory distress syndrome (ARDS) caused by SARS-CoV infection and could represent a useful tool in a multimodal therapy aimed at establishing a rapid anti-inflammatory and antiviral effect.
2. Inflammation Caused by SARS-CoV
In COVID-19 patients, the rapid evolution of the disease requires prompt treatment because only a narrow time window exists for therapeutic intervention. Indeed, after an incubation period, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) starts a rapid replication in the lung airway and alveolar epithelial cells. This activates an immune response with cytokine production, excessive inflammation, and further amplification of the immune response that triggers the cytokine storm. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) may arise with dire consequences (Figure 1). Moreover, the circulation of cytokines to other organs can lead to multi-organ damage.
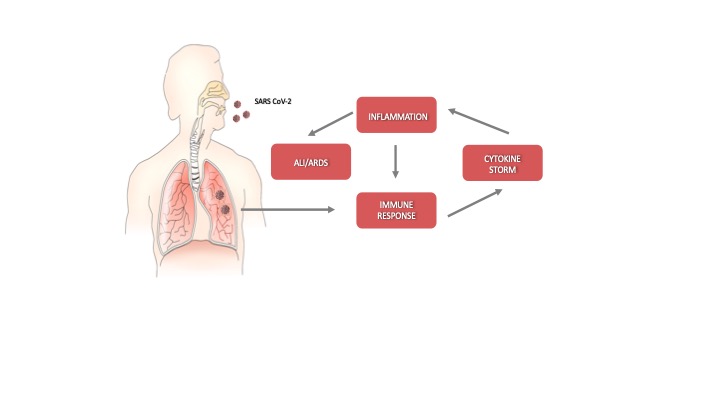
Figure 1. Schematic representation of the progression of coronavirus disease-19 (C)VID-19).
In this regard, pulmonary drug delivery offers the advantage of carrying the bioactive molecule in direct contact with the pathological lung epithelia, thus ensuring a rapid onset of the therapeutic response. High local drug concentrations may be easily obtained by pulmonary administration with a concomitant increase of the pharmacological effect but without the side effects elicited by other administration routes.
Drugs administered by enteral or parenteral routes, indeed, need to reach the blood circulation to be distributed to tissues and organs and enter the pathological site. This general distribution may provide extensive side effects, particularly when high drug administration doses are required to achieve a therapeutically active concentration of the drug in the pathological site.
The increased pharmacological effect provided by high drug local concentrations and the decreased toxicity due to lack of a systemic distribution may improve the overall therapeutic efficiency of the drugs administered by pulmonary route, as demonstrated in several respiratory diseases, such as asthma, cystic fibrosis, and chronic obstructive pulmonary disease [19].
The inhalation devices play a crucial role in the effectiveness of pulmonary drug administration. The most common devices are nebulizers (e.g., jet nebulizers, ultrasonic nebulizers, and vibrating mesh nebulizers), metered-dose inhalers, and dry powder inhalers [20]. The selection of the inhalation device depends on the physicochemical characteristics of the drug and its formulation. Liquid formulations are administered by nebulizers and metered-dose inhalers, and solid formulations by dry powder inhalers. In each case, the inhalation device provides aerosol particles whose size can control the extent of inhaled drug accumulation and the site of drug deposition within the airways. Smaller particles achieve a greater total drug accumulation in the lungs and farther distal airway penetration compared with larger particles. Particles smaller than 5 μm in diameter may flow in the airstream beyond the retro-pharynx and reach the trachea. Particles of 2–5 μm in diameter are deposited in the upper respiratory tract at the level of the trachea and tracheal bifurcation. Particles smaller than 2 μm in diameter deposit in the lower airway and alveolar epithelia [21][22]. Then, the modulation of intrapulmonary deposition through the control of the aerosol particle size can appreciably improve the inhalation drug therapy (Figure 2). The pulmonary administration of drugs mainly provides a local therapy but may also provide a systemic therapy when the physicochemical characteristics of the drugs can support their absorption through the alveolar epithelium at extents suitable to elicit systemic effects. Indeed, the large surface area, extensive vascularization, and single-cell barrier in the alveoli make the lungs an appropriate portal for the systemic absorption of molecules, such as insulin, human growth hormone, etc. [23]
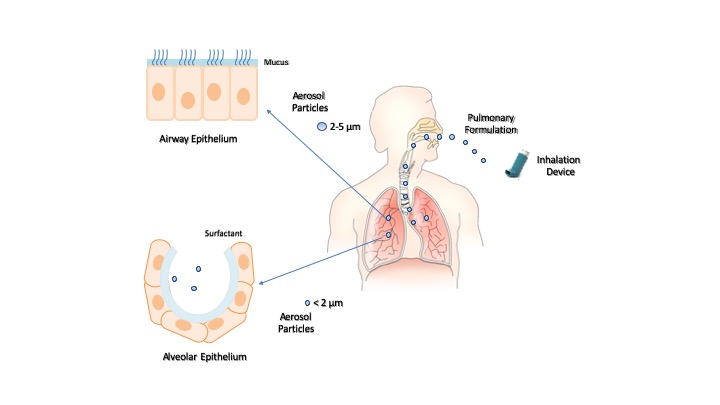
Figure 2. Inhalation of pulmonary formulations and size-dependent distribution of aerosol particles in the respiratory tract.
Pulmonary delivery of fenretinide could be a valuable tool in COVID-19 due to the possibility of obtaining a very high drug concentration in the airway and alveolar epithelia, thus triggering a rapid onset of local anti-inflammatory response. At the same time, the ability of fenretinide to induce an “antiviral environment” could further enhance its therapeutic efficacy (Figure 3).
Figure 3. The potential use of fenretinide in COVID-19 by pulmonary delivery. SARS-CoV-2 lung infection triggers excessive inflammation and activation of the cytokine storm. Pulmonary delivery of fenretinide can provide high drug concentrations in the lung airway and alveolar epithelia, thus inducing a rapid onset of anti-inflammatory activity and an “antiviral environment”. This generates a supportive adjuvant localized therapy useful in multimodal treatments.
In order to be effective, pulmonary fenretinide formulations should provide aerosol particle size smaller than 2 μm, for deposit in the lower airway and alveolar epithelia, where the infection process is amplified by the extensive vascularization. Moreover, after deposition, they should trigger a rapid drug release to speed up the onset of the therapeutic activity.
Such formulations require fenretinide solubilization in an aqueous phase, and the adequate solubilization degree to provide high concentrations of the bioavailable drug, in the lungs, after inhalation.
Unfortunately, the hydrophobic character of fenretinide strongly hinders its aqueous solubilization. Moreover, the possibility to use solubilizing agents, such as tensides or water-mixable co-solvents, is severely restricted, by tolerability issues, in the formulations destined to inflamed lungs.
Highly tolerated, aqueous fenretinide formulations have been obtained by complexation with cyclodextrins [24] or encapsulation in nanomicelles [25].
Complexation with 2-hydroxypropyl beta-cyclodextrin has increased fenretinide aqueous solubility from 0.017 mg/mL (pure drug) to 2.41 mg/mL (complex). The aqueous formulation of the complexed drug, administered by the parenteral route, was well-tolerated and increased the drug bioavailability and antitumor activity in mouse models of different tumor types [24].
Nanoencapsulation in phosphatidylcholine-glyceryltributyrate nanomicelles has increased fenretinide’s aqueous solubilization up to 3.88 mg/mL (nanoencapsulated drug). The intravenous administration of the nanomicelles in mice bearing tumor xenografts showed enhanced drug bioavailability and antitumor activity [25]. Moreover, high tolerability was demonstrated by the absence of adverse effects after repeated administrations and for protracted periods of time.
References
- Ren, L.-L.; Wang, Y.-M.; Wu, Z.-Q.; Xiang, Z.-C.; Guo, L.; Xu, T.; Jiang, Y.-Z.; Xiong, Y.; Li, Y.-J.; Li, X.-W.; et al. Identification of a novel coronavirus causing severe pneumonia in human. Chin. Med. J. 2020, 133, 1015–1024.
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. BioRxiv 2020. preprint.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools [published online ahead of print, 2020 Mar 3]. Virol Sin. 2020.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Mody, N.; McIlroy, G.D. The mechanisms of Fenretinide-mediated anti-cancer activity and prevention of obesity and type-2 diabetes. Biochem. Pharmacol. 2014, 91, 277–286.
- Cooper, J.P.; Reynolds, C.P.; Cho, H.; Kang, M.H. Clinical development of fenretinide as an antineoplastic drug: Pharmacology perspectives. Exp. Boil. Med. 2017, 242, 1178–1184.
- Garaventa, A.; Luksch, R.; Piccolo, M.S.L.; Cavadini, E.; Montaldo, P.G.; Pizzitola, M.R.; Boni, L.; Ponzoni, M.; DeCensi, A.; De Bernardi, B.; et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin. Cancer Res. 2003, 9, 2032–2039.
- Maurer, B.J.; Kang, M.H.; Villablanca, J.G.; Janeba, J.; Groshen, S.; Matthay, K.K.; Sondel, P.M.; Maris, J.M.; Jackson, H.A.; Goodarzian, F.; et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: A report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr. Blood Cancer 2013, 60, 1801–1808.
- Moore, M.M.; Stockler, M.R.; Lim, R.; Mok, T.S.; Millward, M.; Boyer, M. A phase II study of fenretinide in patients with hormone refractory prostate cancer: A trial of the Cancer Therapeutics Research Group. Cancer Chemother. Pharmacol. 2010, 66, 845–850.
- Schneider, B.J.; Worden, F.P.; Gadgeel, S.; Parchment, R.E.; Hodges, C.M.; Zwiebel, J.; Dunn, R.L.; Wozniak, A.J.; Kraut, M.J.; Kalemkerian, G.P. Phase II trial of fenretinide (NSC 374551) in patients with recurrent small cell lung cancer. Investig. New Drugs 2009, 27, 571–578.
- Veronesi, U.; Mariani, L.; DeCensi, A.; Formelli, F.; Camerini, T.; Miceli, R.; Di Mauro, M.G.; Costa, A.; Marubini, E.; Sporn, M.B.; et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006, 17, 1065–1071.
- Villablanca, J.; London, W.B.; Naranjo, A.; McGrady, P.; Ames, M.M.; Reid, J.M.; McGovern, R.M.; Buhrow, S.A.; Jackson, H.; Stranzinger, E.; et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: A report from the Children’s Oncology Group. Clin. Cancer Res. 2011, 17, 6858–6866.
- Reynolds, C.P.; Frgala, T.; Tsao-Wei, D.D.; Groshen, S.; Morgan, R.; McNamara, M.; Scudder, S.; Zwiebel, J.A.; Lenz, H.J.; Garcia, A.A. High plasma levels of fenretinide (4-HPR) were associated with improved outcome in a phase II study of recurrent ovarian cancer: A study by the California Cancer Consortium. J. Clin. Oncol. 2007, 25, 5555.
- Puduvalli, V.K.; Yung, W.A.; Hess, K.R.; Kuhn, J.G.; Groves, M.D.; Levin, V.A.; Zwiebel, J.; Chang, S.M.; Cloughesy, T.F.; Junck, L.; et al. Phase II Study of Fenretinide (NSC 374551) in Adults With Recurrent Malignant Gliomas: A North American Brain Tumor Consortium Study. J. Clin. Oncol. 2004, 22, 4282–4289.
- Vaishampayan, U.; Heilbrun, L.K.; Parchment, R.E.; Jain, V.; Zwiebel, J.; Boinpally, R.R.; Lorusso, P.; Hussain, M. Phase II trial of fenretinide in advanced renal carcinoma. Investig. New Drugs 2005, 23, 179–185.
- Villablanca, J.; Krailo, M.D.; Ames, M.M.; Reid, J.M.; Reaman, G.H.; Reynolds, C.P. Phase I Trial of Oral Fenretinide in Children With High-Risk Solid Tumors: A Report From the Children’s Oncology Group (CCG 09709). J. Clin. Oncol. 2006, 24, 3423–3430.
- Jasti, B.R.; LoRusso, P.M.; Parchment, R.E.; Wozniak, A.J.; Flaherty, L.E.; Shields, A.F. Phase I clinical trial of fenretinide (NSC374551) in advanced solid tumors. Proc. Am. Soc. Clin. Oncol. 2001, 20, 122a.
- Mafalda A. Videira; Jordi Llop; Carolina Sousa; Bruna Kreutzer; Unai Cossío; Ben Forbes; Isabel Vieira; Nuno Gil; Beatriz Silva-Lima; Pulmonary Administration: Strengthening the Value of Therapeutic Proximity.. Frontiers in Medicine 2020, 7, 50, 10.3389/fmed.2020.00050.
- Gupta, V.K.; Bahia, J.S.; Maheshwari, A.; Arora, S.; Gupta, V.; Nohria, S; To study the attitudes, beliefs and perceptions regarding the use of inhalers among patients of obstructive pulmonary diseases and in the general population in Punjab. J. Clin. Diagn. Res. 2011, 5, 434–439.
- Sanchis, J.; Pedersen, S.; on behalf of the ADMIT Team. Systematic review of errors in inhaler use: Has patient technique improved over time? Chest 2016, 150, 394–406.
- Stephen P Newman; Drug delivery to the lungs: challenges and opportunities. Therapeutic Delivery 2017, 8, 647-661, 10.4155/tde-2017-0037.
- Van Heeke, G.; Allosery, K.; De Brabandere, V.; De Smedt, T.; Detalle, L.; De Fougerolles, A. Nanobodies® † Nanobody is a registered trademark of Ablynx NV. As inhaled biotherapeutics for lung diseases. Pharmacol. Ther. 2017, 169, 47–56.
- Isabella Orienti; Federica Francescangeli; Maria Laura De Angelis; Katia Fecchi; Lucilla Bongiorno-Borbone; Michele Signore; Angelo Peschiaroli; Alessandra Boe; Alessandro Bruselles; Angelita Costantino; et al.Adriana EramoValentina SalvatiGiovanni SettePaola ContavalliLello ZollaToshihiko OkiToshio KitamuraMassimo SpadaAlessandro GiulianiMarta BaiocchiFilippo La TorreGerry MelinoMarco TartagliaRuggero De MariaAnn Zeuner A new bioavailable fenretinide formulation with antiproliferative, antimetabolic, and cytotoxic effects on solid tumors.. Cell Death & Disease 2019, 10, 529, 10.1038/s41419-019-1775-y.
- Isabella Orienti; Ferro Nguyen; Peng Guan; Venkatadri Kolla; Natalia Calonghi; Giovanna Farruggia; Michael Chorny; Garrett M. Brodeur; A Novel Nanomicellar Combination of Fenretinide and Lenalidomide Shows Marked Antitumor Activity in a Neuroblastoma Xenograft Model.. Drug Design, Development and Therapy 2019, 13, 4305-4319, 10.2147/DDDT.S221909.




