In the late 1980s, the routes for the formation of apocarotenoids were poorly understood. However, their chemical structure and studies carried out analysing volatiles produced during the ripening of mutant tomato varieties accumulating unusual carotenoids indicated that apocarotenoids were likely derived from the oxidative carotenoid cleavage
[1].
In the years following, a family of carotenoid cleavage dioxygenases (CCDs) that are able to cleave carotenoid at an assortment of double bonds were identified
[2]. The first enzyme of the CCD family was identified from
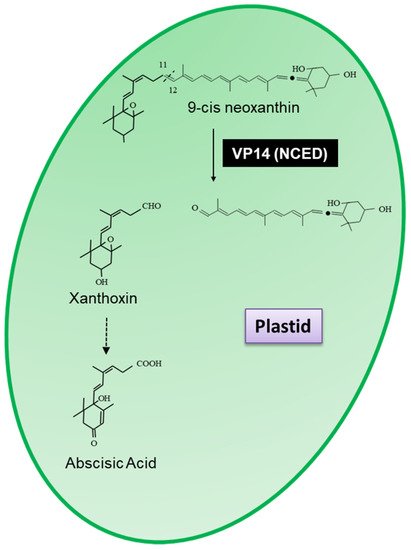
Arabidopsis thaliana (Arabidopsis) and named VP14 (EC.1.13.11.51), which was shown to cleave 9-cis neoxanthin at the 11,12 double bond to form xanthoxin, the precursor of abscisic acid (
Figure 1)
[3][4].
Figure 1. Scheme for the 11,12-cleavage reaction catalysed by VP14 (9-cis-epoxycarotenoid dioxygenase) resulting in the formation of xanthoxin, the precursor of abscisic acid.
Tan et al. [5] identified nine members of the VP14 family in Arabidopsis, five of which have been shown to cleave neoxanthin at the 11,12 double bond and have thus been renamed as neoxanthin cleavage dioxygenases (NCED2, NCED3, NCED5, NCED6(VP14) and NCED9). These enzymes have been extensively studied and are involved in the biosynthesis of the phytohormone abscisic acid (ABA). ABA regulates plant growth, development and stress responses and plays essential roles in multiple physiological processes, including leaf senescence, osmotic regulation, stomatal closure, bud dormancy, root formation, seed germination and growth inhibition among others. The four remaining NCED were shown to cleave a variety of carotenoids generating a variety of (di)aldehydes and ketones and were renamed carotenoid cleavage dioxygenases/oxygenases (CCD1 (EC.1.13.11.71), CCD4 (EC.1.13.11.n4), CCD7 (EC.1.13.11.68) and CCD8 (EC.1.13.11.69)).
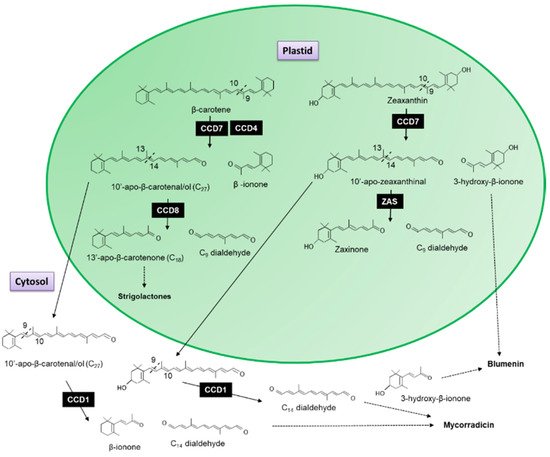
The recombinant CCD7 protein from Arabidopsis exhibited a 9′-10′ asymmetrical cleavage activity converting β-carotene into β-ionone (9-apo-β-caroten-9-one) and 10-apo-β-carotenal (C
27 compound;
Figure 2)
[5]. When the AtCCD8 gene was expressed in
Escherichia coli with AtCCD7, the 10-apo-β-carotenal was subsequently cleaved into 13-apo-β-carotenone and a C
9 dialdehyde
[5]. Since no cleavage activity has been associated with CCD8 when it has been expressed in carotenoid accumulating
E. coli lines to date, Schwartz et al.
[5] concluded that CCD8 functions as an apocarotenoid cleavage enzyme working sequential with CCD7 as the first steps in the formation of 13-apo-β-carotenone.
Figure 2. Scheme for the 9,10-cleavage of β-carotene and zeaxanthin catalysed by CCD7 and CCD4 into β-ionone and 10-apo-β-carotenal (C27 compound) and 3-hydroxy-β-ionone and 10-apo-β-zeaxanthinal (C27 compound). The 13,14 cleavage by CDD8 resulting in the formation of 9-apo-β-caroten-9-one (C9 dialdehyde) and the 13-apo-β-carotenone, the precursor of strigolactones. The 13,14 cleavage of 10-apo-β-zeaxanthinal by Zaxinone Synthase (ZAS) forms Zaxinone and the C9 dialdehyde. The C27 compound is cleaved by CCD1 in the cytosol into β-ionone, 3-hydroxy-β-ionone and rosafluene-dialdehyde (C14 dialdehyde—see Figure 3), the precursor for mycorradicin.
Figure 3. Scheme for the 9,10(9′,10′,) reactions catalysed by the recombinant CCD1 proteins with various substrates. C14 rosafluene-dialdehyde (4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dial). CCD1 activities are carried out in the cytosol.
2. Carotenoid Cleavage Dioxygenase 1 (CCD1) Enzymes Cleave a Broad Category of Carotenoids and Apocarotenoids at Multiple Double Bonds in the Cytosol
It has been shown that this sequential cleavage is the origin of the biosynthesis of strigolactones, a new class of plant hormones essential for plant development (
Figure 2; for review see
[6][7][8][9]). The recently characterized Zaxinone Synthase (ZAS), previously classed as CCD8b, cleaves 3-OH-all trans-β-apo-10′-carotenal (apo-10′-zeaxanthinal), the C27 cleavage product of zeaxanthin into zaxinone (3-OH-all-trans-apo-13-carotenone), a metabolite that regulates strigolactone biosynthesis in rice and the C
9 dialdehyde
[10]. In contrast, CCD1 and CCD4 enzymes have been shown to catabolize a variety of carotenoids and produce volatile and non-volatile apocarotenoids, which are important for the aromas and flavours of flowers and fruits. The following sections of this review will focus exclusively on these two CCDs.
The majority of CCDs/NCEDs have been shown to reside within plastids. The one exception is CCD1, which acts in the cytosol to generate C
13 and C
14 apocarotenoids
[11][12]; however, we cannot rule out a tight association with the outer envelope, as has been reported, for the tomato hydroperoxide lyase
[13], which would potentially give CCD1 access to carotenoids stored in the envelope
[11][12]. Significant amounts of β-carotene have been identified in the outer envelopes of spinach (
Spinacia oleracea;
[14]) and pea (
Pisum sativum;
[15]) chloroplasts, where they have been reported to stabilize chloroplast membranes
[16].
Orthologs of AtCCD1 have been identified in a number of species, including tomato (
Solanum lycopersicum) [11], strawberry (
Fragaria vesca)
[17], petunia (
Petunia hybrida)
[18], pepper (
Capsicum annum L.)
[19], coffee (
Coffea canephora/C. arabica) [20][21], carrot (
Daucus carota L.)
[22], rice (
Oryza sativa)
[23], melon (
Cucumis melo)
[24], fig (
Ficus carica)
[25], grape (
Vitis vinifera)
[26][27][28], rapeseed (
Brassica napus)
[29], and roses (
Rosa damascena)
[19]. CCD1 has been shown to cleave both cyclic and acyclic carotenoids
[30][11][23][31] forming apocarotenoid aldehydes and ketones, indicating that CCD1 may have a more complex reaction tunnel than other CCDs that cleave either cyclic carotenoids or apocarotenoids alone. Using AtCCD1, beta-apo-8′-carotenal as a substrate, isotope labelling experiments have shown that these cleavage activities arise due to a dioxygenase mechanism
[32] requiring only Fe
2 as a cofactor.
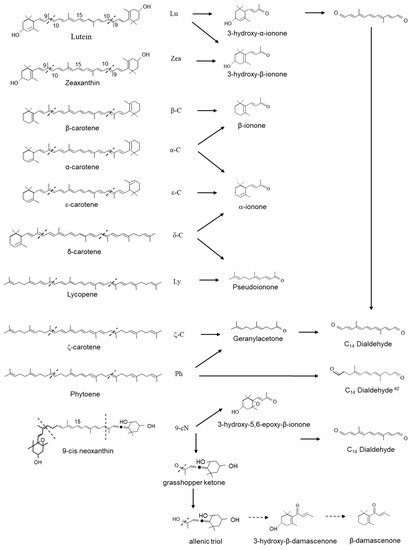
Recombinant CCD1 enzyme and assayed multiple carotenoid substrates in vitro and characterized the cleavage products by thin-layer chromatography and HPLC. CCD1s have been shown to symmetrically cleave the 9,10(9′,10′) double bonds of a large category of carotenoids to form two C
13 compounds and a central C
14 rosafluene-dialdehyde (4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dial) (
Figure 3)
[30][11][33] In assays containing lutein, zeaxanthin and β-carotene, 3-hydroxy-α-ionone (3-hydroxy-9-apo-α-caroten-9-one); 3-hydroxy-β-ionone (3-hydroxy-9-apo-β-caroten-9-one) and β-ionone are formed, whereas α-carotene led to the production of both β-ionone and α-ionone and ε-carotene formed α-ionone (9-apo-α-caroten-9-one) (
Figure 3).
CCD1 has also been shown to cleave nonlinear carotenoids, δ-carotene yields α-ionone (9-apo-α-caroten-9-one) and pseudoionone (6,10-dimethyl-3,5,9-undecatrien-2-one); lycopene yields pseudoionone. Several linear carotenoids, including phytoene and ζ-carotene, are thought to be the precursors of geranylacetone (6,10-dimethyl-5,9-undecatrien-2-one), an important flavour volatile in tomato fruit, as well as precursors for a second C14 dialdehyde (4,9-dimethyldodeca-4,6,8-triendial). Finally, in assays containing violaxanthin or neoxanthin, 5′6-epoxy-3-hydroxy-β-ionone (5,6-epoxy-3-hydroxy-9-apo-β-caroten-9-one) was formed.
In assays containing neoxanthin, the asymmetric cleavage yielded a C
27 allenic-apocarotenal and the C
13 grasshopper ketone (3,5-dihdroxy-6,7-didehydro-9-apo-β-caroten-9-one). The grasshopper ketone is postulated to be the precursor for the formation of the potent odorants β-damascenone (1-2,6,6-trimethyl-1,3-cyclohexadien-1-yl-2-buten-1-one) and 3-hydroxy-β-damascenone (3-hydroxy-1-2,6,6-trimethyl-1,3-cyclohexadien-1-yl-2-buten-1-one) (
Figure 3)
[34][35]. β-damascenone has been shown to be formed from 9′-cis-neoxanthin by peroxyacetic acid oxidation and two-phase thermal degradation without the involvement of enzymatic activity
[35]. Skouroumounis et al.
[36] studied the possible hydrolytic pathway of β-damascenone and suggested formation and determined that allenic triol was the key intermediate.
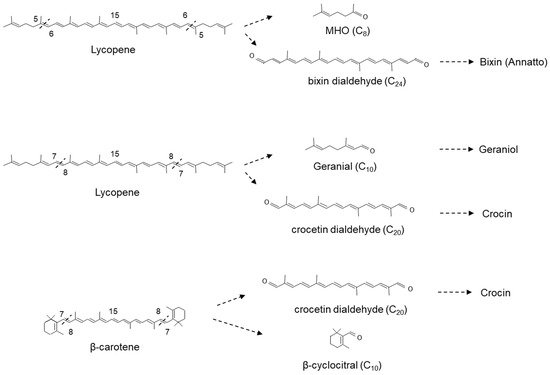
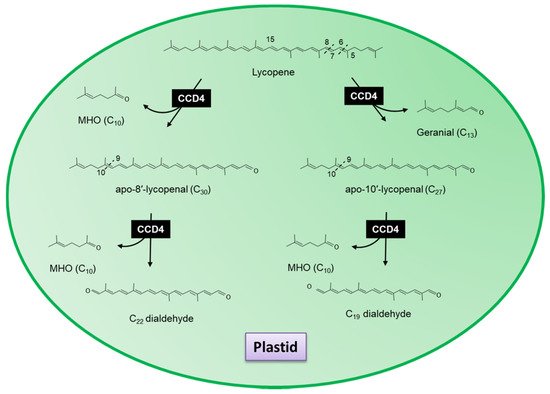
CCD1s from tomato and maize (
Zea maize) have also been shown to cleave the 5,6(5′,6′) double bonds of lycopene in vitro generating the apocarotenoid 6-methyl-5-hepten-2-one (MHO;
Figure 4)
[31]. Furthermore, Ilg et al.
[23], using both in vitro and in vivo assays, demonstrated that the activity of OsCCD1 converts lycopene into pseudoionone (
Figure 3) and MHO (
Figure 4), suggesting that the 7,8(7′,8′) double bonds of acyclic carotenoid ends constitute a novel cleavage site for the CCD1 plant subfamily. Carballo-Conejo et al.
[37] also identified a CCD1 lycopene-specific 5,6(5′,6′)-cleavage dioxygenase (BoCCD1-1) from
Bixa orellana, responsible for the formation of bixin dialdehyde and MHO
[38][39]. Bixin dialdehyde is the precursor for the formation of the dye bixin/annatto (
Figure 4). BoCCD1-1 gene expression correlated with bixin accumulation in
B. orellana [39], suggesting that BoCCD1-1 and its homologue BoCCD1-2 could be involved in the cleavage of lycopene in seed to form bixin. However, data from a study by Cárdenas-Conejo et al.
[37][38] indicated that although BoCCD1-1 is expressed in immature seed, it is also expressed in green tissue (leaf), and BoCCD1-2 was preferentially expressed in leaf. These authors also identified two additional CCD1′s, BoCCD1-3 and BoCCD1-4, which were shown to be expressed in immature seeds at 1.5-fold and 10-fold the levels found in leaf, respectively suggesting that BoCCD1-3 and BoCCD1-4 are more likely involved in the cleavage of lycopene to form bixin dialdehyde in the seed (
Figure 4).
Figure 4. Scheme for the 5,6(5′,6′,) and 7,8(7′,8′,) reactions catalysed by the recombinant carotenoid cleavage deoxygenases (MHO; 6-methyl-5-hepten-2-one) carried out by CCD1 in the cytosol.
Meng et al. [44] showed that VvCCD1 also cleaved β-carotene at the 7,8(7′,8′) position into β-cyclocitral, an important flavour and aroma compound in planta. Interestingly, OsCCD1 was also shown to cleave the 7,8(7′,8′) double bonds of lycopene to form geranial (
Figure 4) [
27].
In the medicinal plant
Catharanthus roseus, the formation of geraniol (isomer of geranial) from geranyl pyrophosphate by geraniol synthase
[40] is a key step in the formation of a number of economically important monoterpene indole alkaloids (MIA). Several of these MIA, such as vinblastine and vincristine, are valuable therapeutic compounds (anticancer drugs:
[41]). CCD1 represents a possible alternate route in the generation of geraniol in planta.
CCD1 has also been shown to cleave apocarotenoids generated by the asymmetric cleavage of a carotenoid.
Medicago truncatula CCD1 antisense plants have been shown to accumulate 10′-apo-β-carotenal/ol (C
27) in root material
[42]. This C
27 dialdehyde is generated by the asymmetric 9′10 cleavage of β-carotene by CCD7, which is subsequently cleaved by CCD8 to form 13-apo-β-carotenone, the precursor of strigolactones (
Figure 2). This indicates that (1) CCD7 result in the accumulation of 10′-apo-β-carotenal/ol, possibly due to a low turnover by CCD8 in the strigolactone pathway; and (2) that CCD1 may act to mop up apocarotenoid generated by previous reactions. Such a role for CCD1 has been previously hypothesized (for review, see Floss et al.
[43]).
The multisite cleavage of lycopene by CCD1 enzymes may be linked to the absence of a terminal ring structure found on the cyclic and oxygenated carotenoids . With no ring, linear carotenoids such as lycopene may penetrate deeper into the reaction tunnel compared to cyclic carotenoids with no stop measure to prevent it. This may well result in a random cleavage pattern and the generation of multiple products from a single linear substrate (
Figure 3 and
Figure 4). The aldehydes and ketones generated by the activity of CCD1 enzymes represent important flavour and fragrance compounds themselves (
Figure 3 and
Figure 4) and act as substrates for the formation of others
[11][31][44][45].
Finally, we also cannot exclude photooxidation as an additional mechanism for the formation of 9′10(9′10′) CDCs β-ionone, pseudoionone, geranylacetone or any of the 5,6(5′6′) and 7,8(7′8′) CDCs generated by the activity of CCD1. It should be noted that the formation of β-ionone from β-carotene by free radical-mediated cleavage of the 9–10 bond has been demonstrated in vitro [51]. In pepper leaves, natural oxidative turnover accounts for as much as 1 mg of carotenoids day-1 g-1 DW [52]. During tomato fruit ripening, carotenoids concentration increases by 10- and 14-fold, mainly due to the accumulation of lycopene [53]. Given the overall quantity of carotenoids that accumulate during fruit ripening, the rates of CDC emission remain very low.
3. Carotenoid Cleavage Dioxygenase 4
Like CCD1, a common feature of CCD4 subset is a 9–10 or 9–10/9′–10′ cleavage activity
[46][47]; however, unlike CCD1, CCD4 is localized to the plastid where it has been detected in the plastoglobules
[47]. Plastoglobules have been identified as a site of carotenoid cleavage by a functionally active CCD4 with β-carotene, lutein and violaxanthin being the principle substrates of CCD4 in vivo
[48]. Huang et al.
[46] cloned CCD4 cDNAs from rose (
Rosa damascena, RdCCD4), chrysanthemum (
Chrysanthemum morifolium, CmCCD4a), apple (
Malus domestica, MdCCD4) and osmanthus (
Osmanthus fragrans, OfCCD4) and expressed them along with AtCCD4 in
Escherichia coli engineered to accumulate carotenoids
[49]. No cleavage products were observed for any of the five CCD4 genes when they were co-expressed in
E.
coli strains that accumulated either cis-ζ-carotene or lycopene. Additional trials using β-carotene as a substrate showed that CmCCD4a and MdCCD4 both cleaved the 9–10 double bond of β-carotene to yield β-ionone; however, OfCCD4, RdCCD4, and AtCCD4 were almost inactive towards this substrate. In the case of RdCCD4 and AtCCD4, the formation of β-ionone was observed in the presence of an apocarotenoid substrate, 8′-apo-β-caroten-8′-al (
Figure 5), while this apocarotenal was barely degraded by MdCCD4, OfCCD4 or CmCCD4a
[46]. It has also been suggested that CCD1 cleaves 10-apo-β-carotenal, a C
27 compound generated by the activity of CCD7 (
Figure 2), suggesting that CCDs also act to further catabolize down-stream products of other CCDs
[43]. From the published data, it also appears that two individual classes of the CCD4 subset exist in planta. Sequence analysis showed that RdCCD4 and AtCCD4 contain no introns, whilst MdCCD, OfCCD4 and CmCCD4a contain one or two introns
[50][46]. It’s interesting to note that the two intronless CCD4s displayed apocarotenoid cleavage dioxygenase activity (ACD) rather than the carotenoid cleavage dioxygenase activity (CCD) observed for the two of the three CCD4s containing introns.
Figure 5. Scheme for the 5,6(5′,6′,) and 7,8(7′,8′,) cleavage of lycopene by CCD4 (MHO; 6-methyl-5-hepten-2-one) and the 9,10(9′,10′,) cleavage of the generated apocarotenoids in a second step.
In Chrysanthemum ‘Jimba’ (
Chrysanthemum morifolium), CmCCD4a contributes to the development of white petals by cleaving carotenoids into their apocarotenoid products
[50]. RNAi interference of CmCCD4a resulted in the development of pale-yellow petals due to the accumulation of carotenoids in the petal tissue
[51]. Brandi et al.
[52] found that CCD4 contributed to the colour of peach flesh and aroma profile, white-fleshed mutants had both a lower carotenoid content and a higher apocarotenoid aroma concentration compared to non-CCD4 expressing yellow flesh peaches, demonstrating the strong link between carotenoids and carotenoid derived aroma volatiles.
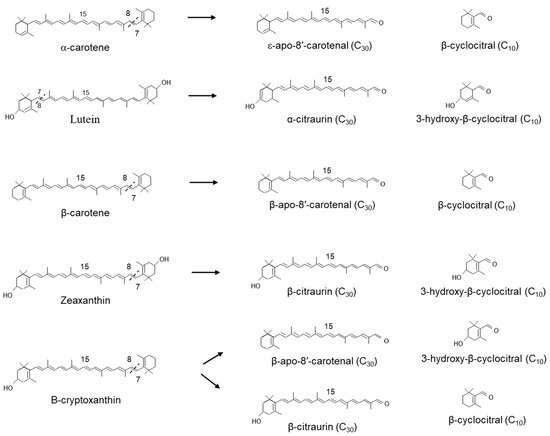
In Satsuma mandarin (
Citrus unshiu), CitCCD4 converts zeaxanthin into 3-hydroxy-β-cyclocitral and the C
30 apocarotenoids β-citraurin (3-hydroxy-β-apo-8′-carotenal), which is responsible for the reddish colour in the peel. CitCCD4 was also shown to use β-cryptoxanthin as an alternate substrate (
Figure 6). However, CitCCD4 cleavage of β-cryptoxanthin generates two possible C
30 apocarotenoids: β-apo-8′-carotenal or β-citraurin, although their relative abundance may indicate that the reaction favours the formation of β-apo-8′-carotenal. In the same experiments, CitCCD4 showed no activity with the substrates, lycopene, α-carotene, β-carotene or violaxanthin
[53].
Related work by Rodrigo et al. in the Washington Navel sweet orange (C. sinensis L. Osbeck), Clemenules mandarin (C. clementina), In silico data mining identified five CCD4-type genes in Citrus. One of these genes, CCD4b1, was expressed in different Citrus species in a pattern correlating with the accumulation of β-citraurin. In contrast to the activity identified for CitCCD4, CCD4b1 was also shown to cleave β-carotene into β-apo-8′-carotenal and β-cyclocitral (Figure 6); α-carotene into one single C30 product, ε-apo-8′-carotenal and β-cyclocitral. When lutein was used as a substrate, only α-citraurin (3-OH-8′-apo-ε-carotenal) was identified, suggesting that 3-hydroxy-β-cyclocitral is also formed. In this instance, Rodrigo et al. showed that CCD4b1 cleaves carotenoid structures with an ε-ring but only on the extremity containing the β-ring. These C30 products of lutein, α-carotene and lycopene are not detected in Citrus extracts, which is not unexpected, as lutein and α-carotene are typical only found in green fruits.
Figure 6. Scheme for the 7,8(7′,8′,) reactions catalysed by the recombinant carotenoid cleavage deoxygenase 4 in the plastid.
When lycopene was used as a substrate, CCD4b1, two different apocarotenoids, apo-10′-lycopenal (C
27) and apo-8′-lycopenal (C
30), were identified to have derived from the 5,6 and 7,8 cleavage, respectively (
Figure 5). CCD4b1 has also been shown to cleave linear apocarotenoids apo-8′-lycopenal and apo-10′-lycopenal at the 5,6 double bonds generating the C
22 and C
19 dialdehydes (
Figure 5)
[54]. These data show that the absence of the ionone ring can substantially alter the cleavage position, as has been suggested for CCD1.
MdCCD4 (
Malus domestica), CmCCD4a (
Chrysanthemum morifolium Ramat), RdCCD4 (
Rosa damascena), OfCCD4 (
Osmanthus fragrans) and AtCCD4 (
A.
thaliana) were all detected in their respective flowers. The expression levels of CCD4 in rose flowers were 42 times higher than those in leaves, indicating that CCD4s may play integral roles in the aroma profile of flowers
[55].
4. Novel Carotenoid Cleavage Dioxygenases
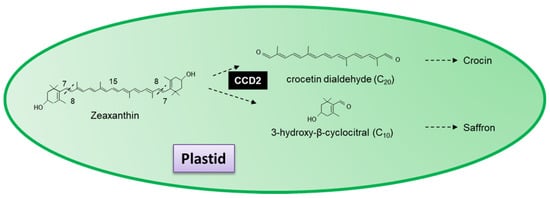
In addition to the nine carotenoid cleavage dioxygenases initially identified, authors have also identified a group of novel cleavage dioxygenases with specific activities. CCD2 is a novel carotenoid cleavage dioxygenase from
C. sativus that catalyses the first dedicated step in saffron and crocin biosynthesis
[56]. Localized in the plastid, CCD2 sequentially cleaves zeaxanthin at the 7,8(7′,8′) forming 3-hydroxy-β-cyclocitral and crocetin dialdehyde, the precursor for the formation of crocin and the spice saffron (
Figure 7)
[56][57]. Ahrazem et al.
[57] demonstrated that CsCCD2 requires a 3-hydroxy-β-ring and does not use β-carotene or lycopene as a substrate. Crocetin dialdehyde has previously been shown to accumulate in the flowers of
Jacquinia angustifolia [58] and the roots of
Coleus forskohlii [59].
Figure 7. Scheme for the 7,8(7′,8′,) reactions catalysed by the recombinant carotenoid cleavage deoxygenases 2 in Crocus sativus.
A second novel carotenoid cleavage enzyme, the
Zea maize CCD10a, cleaved neoxanthin, violaxanthin, antheraxanthin, lutein, zeaxanthin and β-carotene in planta at 5,6(5′,6′) and 9,10(9′,10′) positions, generating MHO (6-methyl-5-hepten-2-one) and the C
13 apocarotenoids, geranylacetone, α-ionone and β-ionone
[60]. ZmCCD10 over-expression and down-regulation led to alterations in the expression of phosphate starvation response regulators (PHR1) and phosphate transporters, whereas down-regulation of CCD10a made plants susceptible to Pi deficiency, over-expression in Arabidopsis enhanced plant tolerance to phosphate limitation
[60], further demonstrating that CCD-generated apocarotenoids have important regulatory functions in planta. Finally, Wang et al.
[10] have reported that CCD10 is found in mycorrhizal plants only. As C
13 apocarotenoids promote arbuscular mycorrhizal symbiosis (
[42][61][62]); over-expression of CCD10 under low phosphate increases C
13 formation, which may promote phosphate acquisition via the mycorrhiza fungal pathway (see
[60]).
5. Apocarotenoids Are Important to Flavour and Aroma
Terpenoid flavour volatiles are generally present in plants at relatively low levels, but possess strong effects on the overall human appreciation of the flavour of
[1][44][63][64][65][66][67][68]. These CDCs are considered to be among the most significant contributors to the flavour and aroma of many fruits and vegetables. For example, α-ionone, β-ionone, β-cyclocitral and β-damascenone have been estimated to contribute as much at 8% to the aroma profile of Valencia orange juice and as much at 78% of the total floral aroma
[69]. Peak levels of β-ionone and geranylacetone emissions from ripe tomato fruit have previously been calculated to be 1.25 pg/g fw
−1 h
−1 and 40 pg/g fw
−1 h
−1, respectively
[44]. Although found in low concentrations compared to other more abundant volatiles such as cis-3-hexenal and hexenal, β-ionone and geranylacetone have much lower odour thresholds, 0.007 nL/L
−1 and 60 nL/L
−1, respectively
[44]. These odour thresholds are more than 10,000-fold lower than other flavour contributing volatiles; thus, these carotenoid-derived volatiles α-ionone, β-ionone, β-cyclocitral and β-damascenone have the potential to greatly impact aroma and flavour, even at low concentrations. Bladwin et al.
[44] determined that β-ionone is the second most important volatile contributor to tomato fruit flavour. The major volatile present in lycopene-containing tomatoes (and watermelons) were geranial, MHO, pseudoionone and geranylacetone, seemingly derived from lycopene
[70]. β-ionone and β-cyclocitral were identified in both tomato and watermelon fruits containing beta-carotene. Furthermore, α-ionone was detected only in an orange-fleshed tomato mutant
Delta, that accumulates ζ-carotene
[70].
β-ionone has also been identified as one of the major components of henna leaves (
Lawsonia inermis L.)
[71], melon
[24] and as a constituent of a number of Moroccan herbs (
Montpellier cistus, Myrtus communis, Cistus ladanifer)
[72], and Yahyaa et al.
[22] identified β-ionone in orange and purple carrot (
Daucus carota) roots. β-ionone has also been shown to be an important contributor to fragrance in the flowers of
Boronia megastigma [73],
Petunai hybrida [18] and
Rosa damascene [74] (see Paparella et al.
[75] for review).
β-damascenone, which was first identified in the oil of the Bulgarian rose (
R.
damascena)
[76], has been described as a potent odorant with an odour threshold of 2 ppt in water
[45]. β-damascenone is one of the most ubiquitous natural odourants, commonly occurring in processed foodstuffs and beverages, where it has also been shown to contribute to the aroma profile of black tea
[77][78], tomato
[64][79], apples
[80], grapefruit juice
[81] and more. It has also been reported to be key component of alcoholic beverages, including wines
[82][83], apple brandy
[84] and beer
[85][86], as well as a primary odorant in Kentucky bourbon
[87]. β-damascenone has also been identified in raw coffee beans
[88], which was not unexpected given the previous identification of carotenoid in raw coffee beans
[21]; however, during the roasting process, β-Damascenone increased strongly
[88].
Another group of volatiles synthesised by CCD1 and CCD1-like enzymes, MHO
[31], and β-cyclocitral
[89] are associated with tomato-like flavour
[90] and sweet floral aroma
[91] of tomato fruit and contributes a sweet/citrus aroma to tomato
[91]. The CCD1-derived geranylacetone and pseudoionone
[11] have also been identified in tomato and contribute to the overall aroma profile. Pseudoionone has been described as having a balsamic-type odour and a floral-type flavour, and geranylacetone has been described as having a floral-type odour and floral-type flavour.
Geranylacetone, α-ionone, β-ionone, β-cyclocitral and β-damascenone were all found in mango fruit
[92]. Interestingly, mango fruit also contained geranial. Whether this accumulation is related to the cleavage of lycopene by CCD1 (
Figure 4) or through the activity of an endogenous geraniol synthase is unknown. As noted above, geranial has also been identified in the headspace of red tomato fruit
[70]. The distillation of apple brandy was also shown to enhance the concentration of two carotenoid-derived flavour compounds, β-damascenone and β-cyclocitral
[84], and MHO has been shown to be present as a component of the floral scent of 50% of all flowering plants analysed
[93], and β-ionone contributes to the aroma profile of petunia flowers
[18]. Baldermann et al.
[94] functionally characterized CCD1 from
Osmanthus fragrans Lour and found it cleaved α- and β-ionone, contributing to the aroma of flowers.