1000/1000
Hot
Most Recent

Alongside their function in primary haemostasis and thrombo-inflammation, platelets are increasingly considered a bridge between mental, immunological and coagulation-related disorders. This review focuses on the link between platelets and the pathophysiology of major depressive disorder (MDD) and its most frequent comorbidities. Platelet- and neuron-shared proteins involved in MDD are functionally described. Platelet-related studies performed in the context of MDD, cardiovascular disease, and major neurodegenerative, neuropsychiatric and neurodevelopmental disorders are transversally presented from an epidemiological, genetic and functional point of view. To provide a complete scenario, we report the analysis of original data on the epidemiological link between platelets and depression symptoms suggesting moderating and interactive effects of sex on this association. Epidemiological and genetic studies discussed suggest that blood platelets might be relevant biomarkers of MDD prediction and occurrence also in the context of MDD comorbidities. Finally, this review has the ambition to formulate some directives and perspectives for future research on this topic.
Platelets are increasingly considered a putative bridge linking mental, immunological, and coagulation-related disorders, alongside their function in primary haemostasis and thrombo-inflammation. Patients with complex neurological and neuropsychiatric disorders such as schizophrenia, Parkinson and Alzheimer’s diseases have been described with altered platelet function, already several decades ago [1][2][3][4][5][6]. Since then, numerous studies have given better insight into those early observations, by deciphering the cellular, molecular and functional similarities between platelets and neurons [7][8][9][10][11], and they have set the basis for the use of blood platelets as a working model to study several neurological diseases [3][8][9][12][13][14][14][15][16].
Platelets not only mirror neurons in several aspects, but directly contribute to the pathophysiology of those diseases affecting them in several ways. We will focus on the link between platelets and the pathophysiology of major depressive disorder (MDD), one of the leading causes of disability and disease burden worldwide. MDD presents as a persistent low mood, with associated changes in behaviour, cognition, sleep and appetite, impaired social and occupational functioning, increased risk of self-harm or suicide [17][18]. MDD is also associated with increased mortality due to several comorbidities, especially cardiovascular disease (CVD). Moreover, depression shows a high comorbidity with many other disabling conditions, including neurodegenerative, neuropsychiatric and neurodevelopmental disorders. With this review we first aim at providing functional evidence for a platelet pathophysiological involvement in depression by highlighting the intrinsic platelet characteristics in drug-free depressed patients. Additionally, we present here an overview of the current state of the art in epidemiological and genetic studies linking MDD and its main co-morbidities with platelets, in addition providing some original data on the epidemiological link between platelet parameters and depression symptoms.
Despite their different embryonic origin [15][19], platelets and neurons share several characteristics. Platelets mimic the stable synaptic structure between neurons, i.e. where they interact with each other [4]. With neurons, they share the complex molecular machinery that regulates granule trafficking [7], controlling the calcium-dependent release reaction of stored agonists, after activation stimulation in platelets and the neurotransmitter release following an action potential in neurons [20]; they both have similar secretory vesicles in terms of content, storing molecules such as serotonin or 5-hydroxytryptamine (5-HT), dopamine, epinephrine, glutamate, gamma-aminobutyric acid (GABA), calcium, adenosine 5′-diphosphate (ADP) and Adenosine 5′-triphosphate (ATP). Platelets and neurons also share a number of proteins that include serotonin transporters and receptors [8][19][20] as well as some markers originally known as neuron-specific such as reelin, amyloid precursor protein (APP) and Brain-Derived Neurotrophic Factor (BDNF) [10][21][22].
Several studies have identified an association of the (epi)genetic variability of genes encoding some of the key players in depression etiology with both mood disorders and thrombo-inflammatory conditions such as CVD. Those studies have been inspired by the functional role that neuron- and platelet- shared proteins have in platelets themselves. In many instances, these genes encode shared proteins between neurons and platelets including the serotonergic pathway, cannabinoid receptor 1 and BDNF [23][24][25][26][27][28]. BDNF is probably the most studied of them as one of its specific variants (Val66Met, G to A, rs6265) has been consistently reported to influence the predisposition to CVD associated with depression [25][27][29]. Although epi(genetic) studies identifying novel genes with pleiotropic effects on both platelets and neurons in the context of depression are missing, there is increasing functional evidence that links some of these proteins to both depression and thrombo-inflammation.
The relation between platelets and depression has been deeply investigated, both through epidemiological and – less often - through statistical genetics approaches. Some studies reported increased platelet activation in individuals with depression, compared to healthy controls. Musselman et al. observed an enhanced baseline platelet activation and responsiveness in patients affected by MDD, as suggested by the increased expression of platelet αIIbβ3 and P-selectin [30], while Pinto and colleagues [31] demonstrated an impairment of L-arginine-nitric oxide signaling in platelets of depressed compared to healthy subjects. Morel-Kopp et al. [32] reported a direct association of MDD with a higher number of CD62- and CD63-positive platelets and excitability, which were attenuated by a 6-month treatment with anti-depressants, in line with previous findings on platelet secretion in response to collagen binding [33]. An increased oxidative stress and hyperaggregability were observed in platelets of MMD cases compared to controls [34], as well as a higher content of serotonin, interleukin 1β, PF4 and CD40 ligand (CD40L) [35]. In line with this evidence, a recent longitudinal study on young males reported mental stress to be associated with increased and prolonged proinflammatory platelet bioactivity: while exposure to chronic stress led to an increased number of CD63+ platelets, acute stress was associated with alterations of CD62P+, CD63+, PAC-1+ platelets and of platelet-leukocyte aggregates [36].
Other epidemiological studies investigated the relation between MDD and platelets by making use of platelet parameters commonly tested, like MPV and Plt. A positive association between MPV and MDD was reported in a Turkish population sample (N = 2,286, 287 cases) [37], and later replicated in a study comparing 103 MDD patients and 106 controls [38], as well as in a hospital-based study (90 cases vs 49 controls) [39]. However, these studies revealed contrasting evidence of association between Plt and MDD status: while Bondade and colleagues observed an increased Plt in depressed patients [39], Cai et al. found no statistical evidence supporting that finding [38]. They reported a positive association between MDD and plateletcrit (PCT), i.e. the product of MPV and Plt, which represents the total mass occupied by platelets in the blood [38]. Platelet parameters have also been studied with reference to MDD treatments: in a small study comparing 15 MDD patients under Escitalopram therapy – one of the most used SSRI treatments – and 17 healthy controls, treated patients exhibited a significant reduction in both MPV and Plt, which were instead higher than in controls at baseline [40]. Another study comparing 31 patients with life-long recurrent depression treated with SSRIs and 31 matched healthy controls, reported significantly higher MPV, platelet distribution width (PDW, an index of size variability of circulating platelets) and platelet-to-larger cell ratio (P-LCR; i.e. the proportion of large platelets with volume >12 fL, which represents an index of platelet size useful in the diagnosis of thrombocytopenia) in depressed participants [41]. Although a direct link between PDW variability and platelet function has not yet been fully established [42], this evidence suggests once again that lower platelet activation and function may be a feature of depression, along with lower platelet and blood plasma serotonin, and lower platelet reactivity. Moreover, studies on collagen- and epinephrine-induced aggregation and the percentage of spiny and discoid platelets also suggested a lower platelet reactivity as a potential feature of depression [41]. In line with this evidence, in a comprehensive analysis of the relation between low-grade inflammation and mental health in a large Italian population cohort (the Moli-sani study; N=12,732), our group identified a significant positive association between continuous depressive symptoms and PDW [43]. This association survived conservative adjustments for several sociodemographic, health and lifestyle covariates, suggesting the existence of shared genetic underpinnings between depressive status and platelet size variability [43].
Depression and depressive symptoms are frequently comorbid with cardiovascular disease, neurodegenerative, neuropsychiatric and neurodevelopmental disorders [44]. The latter have important clinical implications since depression may dramatically contribute to worsen those diseases and in general have an impact on overall health [45]. Indeed, comorbid depression has been associated with worse prognosis and increased mortality [46][47] and with a higher risk of developing other diseases later in life [48]. Several mechanisms have been proposed to explain the co-occurrence of depression with the comorbidities mentioned above, including treatment-induced morbidity, behavioral and psychological factors, but also underlying biological processes [44]. Depression and depressive symptoms have been established already for a long time as important risk factors for cardiovascular disease (CVD) related mortality and occurrence. Increasing evidence has pointed to a specific role for platelets in influencing the CVD-MDD comorbidity [49][50][51]. First of all, a higher platelet aggregability has been considered as a marker of patients with both CVD and MDD, as platelet hyperactivity could explain both pathological phenotypes [50][52]. As highlighted previously, higher platelet aggregability is a signature of depressed patients without cardiovascular events. Depressed patients display increased platelet serotonin receptor concentrations [53][54] and abnormally low platelet SERT levels [55] which would result in elevated serotonin concentration in the bloodstream. This would in turn lead to abnormal platelet aggregation in atherosclerotic arteries [56][57]. Indeed, elevated blood levels of serotonin are predictive of CAD and ischemic cardiac events in patients with suspected CAD [58], and in vitro experiments have demonstrated higher platelet aggregability in CVD/MDD patients [59][60][61][62]. In addition to that, post-myocardial depressed patients showed abnormal whole blood and platelet serotonin levels [59], and depressed CVD patients have higher serotonin receptor density [63]. Anxiety, often accompanying depression (see below), has been shown to be a predictor of adrenaline and serotonin-dependent platelet reactivity in CAD patients [64]. Both PF4 and βTG have been investigated in this context and have been shown to be higher in depressed compared to non-depressed CAD patients [60][61][65], as well as in depressed CAD patients compared to CAD- and depression-free controls [66].
Common neurodegenerative diseases linked to the accumulation of neurotoxic protein aggregates are usually diagnosed when the disease is already at an advanced stage of neurodegeneration [12][67]. This makes it very important to identify potential biomarkers that are easy to measure and that could predict the incident risk of these disorders, e.g. as circulating biomarkers [68]. Platelets are in different ways associated with the pathophysiology of neurodegenerative disorders. First they have a crucial role in the metabolism and storage of dopamine, Aβ peptides and APP [10], as previously discussed. This led the way to a handful of functional platelet studies briefly reviewed here below (most relevant human studies) and more extensively elsewhere [69]. More recently, the link between neurodegenerative disorders and classical blood platelet parameters like MPV, Plt and PDW has been investigated also at the epidemiological level, in a relatively limited number of studies. Indeed, platelet indices are easy-to-measure and standardized across laboratories and could be used as good prediction and/or prognosis markers of neurodegenerative risk.
Among psychiatric comorbidities of MDD, panic disorder (PanDis) [70][71][72][73][74][75][76], Generalized Anxiety Disorder (GAD) [39][77], Post-traumatic Stress Disorder (PTSD) [78][79] all have been investigated with reference to platelet parameters. Neuropsychiatric conditions, still, present differences in platelet parameters also when compared among themselves. Wysokiński & Szczepocka [80] compared platelet parameters like Plt, MPV and P-LCR among 2,377 subjects including schizophrenia, depression, bipolar disorder and mania patients and identified several differences among these groups, for all the markers tested.
Among neurodevelopmental disorders, alterations in platelet parameters have been associated with important conditions like Autism Spectrum Disorder (ASD) (as reviewed in [9]) and Attention Deficit Hyperactivity Disorder (ADHD) [81][82][83][84][85].
5. Conclusions
To summarize, platelet pathophysiology has strong implications in the occurrence of MDD and of its related co-morbidities and they support the view that platelets reflect a circulating form of neurons [11]. However, several aspects in this fascinating hypothesis need yet to be disentangled.
First of all, the shared genetic bases between platelet variability and MDD, despite some first attempts [42][86], still needs to be elucidated through larger genetic epidemiology studies. The latter should take into account important features such as gender differences that are known to be linked to both platelet variability [87] and to the occurrence of MDD risk [88]. More studies that consider the occurrence of certain MDD comorbidities in specific population subgroups (i.e. women and pregnant women, children, aging population) are still lacking but are utterly important for further investigations.
Second, and related to the first point, the influence of sex on the relationship between depression risk/symptoms and platelet parameters needs to be clarified, following-up on the relevant evidence of moderate and interactive effects which we have reported here for the first time. Additional studies are warranted to verify whether the differential associations in women and men between platelet activation parameters and MDD results from intrinsic sex-specific (hormonal) differences influencing platelet activation or also from MDD-related factors.
Third, epidemiological and genetic studies increasingly identify PDW as an interesting yet functionally poorly understood candidate biomarker to test in terms of prediction of MDD and related neuropsychiatric/neurodegenerative risk in the future. The largely concordant evidence reported here that links PDW to MDD, ADHD, PanDis, as well as to cognitive traits and disorders like AD (Figure 1), is an incentive to further investigate this marker and its clinical implications. A particular topic of investigation may be its different associations and genetic correlations with PD and AD, two common neurodegenerative disorders with partly shared biological basis.
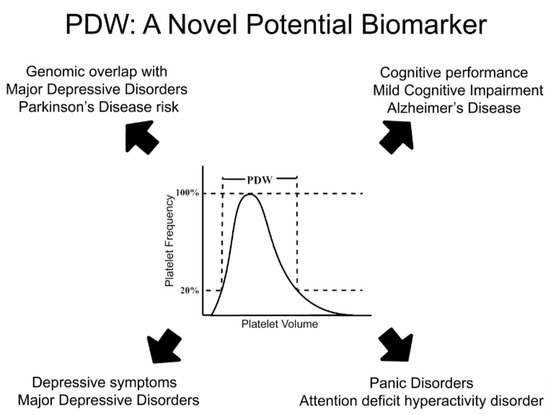
Figure 1. PDW: a novel potential biomarker for depression, its neurodegenerative and psychiatric comorbidities. Platelet distribution width (PDW) represents and index of platelet volume variability in a subject. It has been associated with both depressive symptoms and major depression, but also with neurodegenerative and psychiatric comorbidities like Alzheimer’s disease, mild cognitive impairment, attention deficit hyperactivity disorder and panic disorder. Beyond epidemiological evidence, genomic studies identified consistent co-heritability based on common genetic variants between PDW and depression, as well as between PDW and Parkinson disease risk. Overall, this evidence suggests PDW as a very promising candidate biomarker for MDD and its comorbidities to investigate in the future.
Lastly, platelet serotonin might not only be important in platelet activation and subsequent involvement in platelet-related pathologies that are co-morbid with MDD, but also for its role as an epigenetic modulator in the serotonylation, a covalent posttranslational modification occurring at the level of histones thereby influencing gene expression [89]. Indeed, epigenetics has been hypothesized to play a major role in the etiology of depression [90][91][92]. In this entry, serotonylation could represent a platelet-mediated link between serotonin and depression onset, a hypothesis which warrants further investigation.
As it appears from these open issues, much remains to be done to clarify the relationship between platelets, depression and its comorbidities in detail. This entry tries to lay out at least some pieces of the puzzle for investigators in the field, having the ambition to expand into future research on this topic.