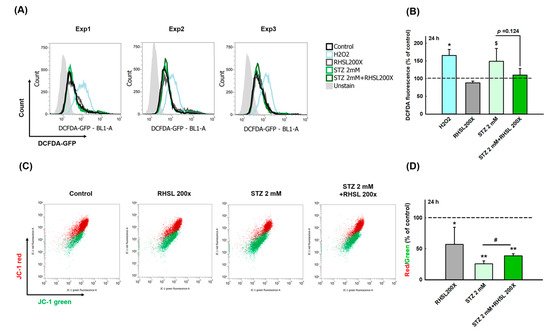
To explore whether RHSL can suppress the cell damage provoked by ROS in STZ-treated RIN-m5F cells, a cellular ROS assay was performed (Figure 2A). As a positive control, H2O2 accelerating the process of beta cell destruction through significantly increasing the ROS production (Figure 2B, p < 0.05). Even though the peak value was too large and there was no significant difference between the two groups, we still observe that STZ increases the ROS production in RIN-m5F cells (Figure 2B, p = 0.066), while RHSL slightly suppresses the ROS production caused by STZ (Figure 2B, p = 0.124).
Next, to investigate the changes of mitochondria membrane potential (ΔΨm) in STZ-treated RIN-m5F cells after RHSL intervention, a JC-1 assay was utilized (
Figure 2C). We observed that the red/green ratio significantly decreased after STZ stimulation (
Figure 2D,
p < 0.001), while RHSL significantly restored the red/green ratio in STZ-treated RIN-m5F cells (
Figure 2D,
p <0.05). Therefore, these results have clearly indicated that RHSL possesses the potential ability to abrogate stress resulted from STZ stimulation.
3. Discussion
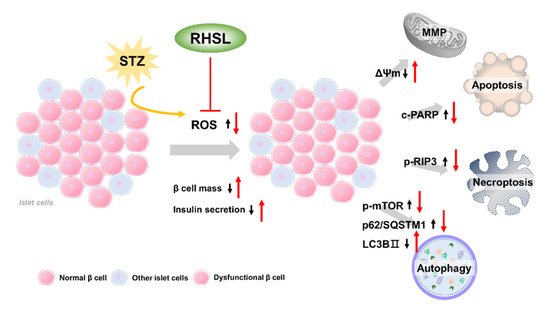
The current study provides new insights regarding the potential utility of rice husk silica liquid (RHSL) in T2DM, RHSL abrogated the effect of streptozotocin (STZ) on the mass of RIN-m5F pancreatic β cells and the ability of insulin secretion, via reducing the reactive oxygen species (ROS) production, recovering the depolarization of mitochondrial membrane potential (ΔΨm), as well as reversing the expression of cleaved-PARP, p-RIP3, p-mTOR, p62/SQSTM1, and LC3B protein. The proposed mechanisms of RHSL action in the prevention of STZ-induced RIN-m5F cell death were summarized in Figure 6.
Figure 6. Schematic model of the potential mechanisms of RHSL protective effect on RIN-m5F cells from STZ-induced oxidative damage. Black arrows are the effects of STZ, red arrows are effects of RHSL. Abbreviation: RHSL, rice husk silica liquid; STZ, streptozotocin; ROS, reactive oxygen species; ΔΨm, mitochondria membrane potential; RIP3, receptor-interacting protein 3; PARP, poly (ADP-ribose) polymerase; mTOR, mammalian target of rapamycin; p62/SQSTM1, sequestosome-1; LC3, light chain 3.
The major pathways associated with STZ-induced pancreatic β cells death include DNA methylation and activation of DNA repair mechanisms
[13], nitric oxide (NO) overproduction
[14], and glucose auto-oxidation which in turn generates free radicals
[13]. Moreover, STZ induces pancreatic β cell toxicity through mitochondrial dysfunction, ROS production, necrosis, and apoptosis
[4].
RIN-m5F cells are a widely used insulin-secreting cell line, mainly containing insulin and a small amount of glucagon and somatostatin
[15]. STZ administration leads to the rapid destruction of pancreatic β cells, thus massive insulin was released from ruptured β cells to blood
[4]. A previous study revealed that a considerable decrease in insulin secretion ability in STZ-treated INS-1 cell, whereas paeoniflorin (PF) pretreatment was capable of improving the insulin secretion ability of INS-1 cells
[16]. In addition, 1,25(OH)
2D3 enhances insulin secretion in STZ-treated d MIN6 cells at low and high glucose concentrations
[17]. An in vivo experiment showed that, compared with the control group of diabetic mice, the plasma insulin of the Si treatment group was significantly reversed to near-normal levels
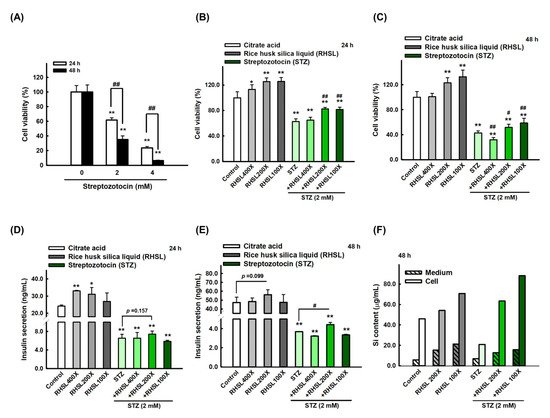
[11]. Similar to the results of previous studies, the insulin levels of pancreatic β cells in the STZ treatment group were significantly reduced in our study, while RHSL significantly reversed the insulin level that was reduced by STZ.
Higher levels of ROS are found in the islets of T2DM patients, and their presence attenuates insulin secretion
[4]. STZ that induces diabetes is also closely related to the production of ROS. Several studies have demonstrated that STZ induces damage predominantly by provoking the production of ROS and NO in islet cells
[4]. In STZ-treated MIN6 β-cells, ROS generation was statistically significantly increased by 55.1 ± 4.7% (
p < 0.001)
[18]. Similarly, AI-Nahdi et al. observed a significant increase in intracellular ROS production in RIN-m5F cells treated with 1 mM STZ for 48 h
[19]. Comparably, our study showed that ROS production was increased in RIN-m5F cells following treatment with 2 mM STZ for 24 h, while RHSL slightly reversed the effects caused by STZ. Therefore, we speculated that RHSL can reverse the cytotoxicity and damage induced by STZ by reducing the content of ROS in RIN-m5F cells.
Under the stimulation of oxidative stress, the mitochondrial membrane potential (ΔΨm) will be changed, which is characterized by the disordered membrane potential leads to the opening of mitochondrial pores, thus releases cytochrome C into the cytoplasm, which in turn triggers the downstream apoptotic cascade
[20][21]. AI-Nahdi et al. observed a significant loss in the membrane potential after treatment with 10 mM STZ for 24 h
[22]. Similarly, our results showed a significant mitochondrial membrane depolarization after treatment with STZ in RIN-m5F cells. Importantly, RHSL treatment showed a significant recovery of the membrane potential in STZ treated cells.
Most studies have shown that pancreatic β cell apoptosis is a key process in the pathogenesis of T1DM and T2DM, so it is important to alleviate or suppress the effect of STZ that induces diabetes. Fucoidan, a brown algae extract, which is used to be explored against diabetic nephropathy, has also been found to reduce the number of apoptotic cells in STZ-treated NIT-1 cell
[23]. In addition, Lianga et al. showed that Fudan-Yueyang G. lucidum alleviated the apoptosis in STZ-treated INS-1 cell
[24]. In our study, STZ induced the expression of apoptosis-related protein PARP, while RHSL reversed these results and significantly reduced the expression of the cleaved-PARP protein. The contradictory research revealed that the ability of nano-SiO2 to induce ROS and promote apoptosis in normal human hepatic L-02 cells
[25], while no significant alteration of ROS levels and cell viability were seen in 48 and 86 nm of SiO2 exposed groups. Therefore, we believe that the size of silicon particles and cell viability are worthy of further research.
Necroptosis is considered to be an alternate cell death mechanism triggered when apoptosis is blocked. Besides the inhibition of caspase-8 prevents apoptosis induction
[26], receptor-interacting protein 3 (RIP3) plays a critical role in the switch between apoptotic and necroptotic cell death triggered by tumor necrosis factor (TNF)
[27]. In general, RIP1 mediated apoptosis does not require the presence of RIP3, while RIP1 is accompanied by high levels of RIP3 that promote the formation of necrosome complexes and switch cell apoptosis into necroptosis
[26]. More specifically, RIP3 increases TNF-induced ROS production via activation of glutamate-ammonia ligase (GLUL), glutamate dehydrogenase 1 (GLUD1), and eventually induce necroptosis
[27][28]. At present, there is no literature to investigate the level of RIP1/RIP3 for pancreatic β cells following induction by STZ, while a previous study indicated that STZ-induced renal injury tissue had significantly higher expression levels of RIP1 and RIP3
[29].
Autophagy is a catabolic process of the lysosomal degradation pathway under conditions of stress, which maintains metabolic turnover and homeostasis in pancreatic β cells
[30]. The autophagy process involves the inhibition of the mammalian target of rapamycin complex 1 (mTORC1) and the deacetylation of autophagy components, which promotes the fusion and degradation of autophagosomes containing substances to be degraded and lysosomes containing acid hydrolases
[30]. Once autophagy is dysregulated, it will also promote the loss of β cell mass and function
[31]. Previous research confirms this argument, they found that autophagy-related (ATG) 7 knockout mice showed decreased β-cell mass through increased apoptosis, which ultimately lead to decreased insulin secretion and impaired glucose tolerance
[32]. The most representative indicators of autophagic flux include light chain (LC)3 (autophagosome marker) and p62/SQSTM1 (autophagy adaptor), the latter being the link between LC3 and the ubiquitinated substrates
[33]. In our study, we found a significant increase in the transition of LC3-I to LC3-II, while p62/SQSTM1 was decreasing after RHSL treatment in STZ-induced RIN-m5F cells, which indicate that RHSL is indeed involved in the process of autophagy regulation.
On the other hand, 3-methyladenine (3-MA) and chloroquine (CQ) are frequently used autophagy inhibitors that function in early autophagy through PI3K inhibition
[34] and in late autophagy by blocking the fusion of autophagosomes and lysosomes
[35], respectively. In our study, we found that RHSL significantly induced the expression of cleaved-PARP in 3-MA-treated RIN-m5F cells, and similar results were also observed in CQ-treated RIN-m5F cells, these results reveal that RHSL protects RIN-m5F cells by inducing autophagy. Similar results have also been observed in previous studies, vitamin D enhanced autophagy while inhibited apoptosis in STZ-treated MIN6 β-cells
[17]. In addition, T. stricta extract was able to significantly reverse the autophagy markers in response to STZ to near normal level in RIN-m5F cells
[36].
The factors that determine the bioavailability of Si element include concentration, type of food, and species in which silicon is present
[37][38]. Si in serum and tissues exists in free form and can be absorbed and eliminated in urine quickly due to free diffusion across cell membranes
[11]. This implies the advantage of long-term intake without excessive retention and accumulation in the body.