1000/1000
Hot
Most Recent

Recent contributions to thermochemical heat storage (TCHS) technology have been reviewed and have revealed that there are four main branches whose mastery could significantly contribute to the field. These are the control of the processes to store or release heat, a perfect understanding and designing of the materials used for each storage process, the good sizing of the reactor, and the mastery of the whole system connected to design an efficient system. The above-mentioned fields constitute a very complex area of investigation, and most of the works focus on one of the branches to deepen their research.
Global energy demand continues to increase with high growth in the exploitation of fossils fuels, whose prices and environmental issues are leading scientists and engineers to find inclusive solutions. The use of renewable energy fits these challenges well. Solar energy, mainly known for its infinite and renewable nature, is one of the most widely used today. Due to its seasonal availability, the major challenge continues to be the need to store solar energy in periods of high availability to be re-used in periods of shortage. Several solar energy storage methods have been developed, among which TCHS appears to be one of the most promising. TCHS processes have the potential to store heat over theoretically infinite time and long-distance transportation. More recently, an increasing number of laboratory-scale as well as large-scale projects are being developed in the field of energy storage for both low-temperature and high-temperature applications, but the technology is still not fully accessible and marketable. To further identify the problems and limits encountered, several synthesis works are published, reviewing the progress of the studies and mentioning the future challenges to be tackled [1][2][3]. Desai et al. [4], in their recent review on TCHS systems for both heating and cooling process applications, have summarized some of these works with their highlights, as shown in Table 1 .
| Author(s) | Highlights | Refs |
|---|---|---|
| N’Tsoukpoe et al., (2009); Tatsidjodoung et al., (2013) |
|
[5][6] |
| Cot-Gores et al., (2012) |
|
[7] |
| Yu. et al., (2013) |
|
[8] |
| Sole et al., (2015); Fopah-Lele and Tamba (2017) |
|
[9][10] |
| Krese et al., (2018) |
|
[11] |
| Lizana et al., (2018) |
|
[12] |
| Kuznik et al., (2018) |
|
[13] |
| Sunku Prasad et al., (2019) |
|
[3] |
| Desai et al., (2020) |
|
[4] |
Many of these works have shown promising results in terms of convertibility of the energy stored by the material, the development of innovative systems that are compact and simple to maintain, the availability of output data and their analysis, and the improvement of the efficiency of the existing system. However, despite the advantages offered by the process, the perspicacity of their scientific approach, and their achievements, they have only reduced the effects of the problems encountered, and several drawbacks remain: the costly investments, the high cost of the storage materials, the limited heat and mass transfer capacity, and the poor heat storage density in the current systems. This review provides a recent state of the art of TCHS technologies, including some key terms and concepts, advances in TCHS systems, and an analysis of some relevant recent research. The most significant findings and perspectives are provided.
Compounds X and Y may be stored separately when the energy is stored, depending on the nature of the compounds and the chemical reactions they involve [14][15]. This storage can be in different forms: either relative to physisorption in adsorption grids (adsorption) or relative to absorption in absorbent solutions or also relative to chemical reactions as chemical potential. Applications such as building space heating, domestic hot water supply, or sterilization of certain work tools may involve the use of sorption heat storage processes while high-temperature heat uses such as in gasification process, the load shifting, the power generation, or electricity production processes may require chemical heat storage using pure chemical reactions to store energy as chemical potentials. Through the literature [16], TCHS processes can be organized as shown in the following diagram in Figure 1 .
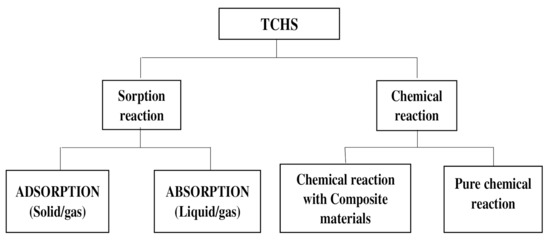
For power generation applications requiring both high working temperatures and high enthalpies of reaction, chemical reaction storage is preferred. In the literature, the chemical reactions concerned are of two types: the coordination reaction of ammonia and the hydration reaction of salt hydrate with water [17][18]. The issue of classifying the coordination reaction as solid sorption or a chemical reaction remains unclear. Wang et al. [19] and N’Tsoukpoe et al. [20] were inclined to use chemical adsorption (chemisorption) to describe the coordination reactions. The reason is possibly due to the importance of the part played by the solid surface in these solid/gas reactions, while Cot Gores et al. [7] have identified these reactions as solid/gas absorption reactions due to the molecular structure transformation that occurred. To avoid possible contestation and ambiguity, the concept of chemical reaction appears to be an acceptable approach [21] and therefore will be adopted here. The reactions occurring are reversible. The chemical reaction involved is as follows: AB + Q ↔ A + B (2)
To overcome the constraints of increasing the capacity of the materials used for pure chemical reactions, a promising new process is being developed and continues to receive improvement and scientific advancement. These are chemical reactions with composite materials [22]. This process is similar to the previous one, but here, the mixture of known standard materials is used with a new material that has specific characteristics for improving heat and mass transfer properties and increasing the durability as well as the stability of such composite material over time [23][24][25].
Research on the development of composite materials is a recent known field of thermochemical heat storage to enhance the heat transfer in thermochemical reactors, and many research structures are interested in it.
A TCHS reactor is a device that contains the storage material and at the same time carries out the process of storing and releasing the energy according to the adopted configuration. Thus, it appears as a crucial component of heat storage processes, and its optimization would allow obtaining very high efficiency of energy storage and restitution. However, few studies have been carried out to develop thermochemical reactors from laboratory prototypes to large-scale project pilots. Those studied and developed so far have a crucial issue of very limited heat and mass transfer in the studied systems [16]. To ensure a sufficient heat and mass exchange inside the reactor, corners where there is no contact between sorbent and sorbate should be avoided or minimized. Indeed, the material selected for storing heat has generally a very high energy density, but the global energy density of the entire system after the storage process remains much lower (generally the third at the end) than that of the materials used [26], which is generally due to the significant heat losses when storing energy. Particular emphasis on all aspects of the reactor (its sizing and optimization) could maximize the storage density of the system, limit energy losses, and thus increase the power of the reactor. TCHS reactor technology consists of two stages, whose mastery could significantly contribute to increasing the maturity of the technology (TRL). These are the microscopic and macroscopic aspects of the design. Most of the published works have focused only on the macroscopic aspect and therefore the published results are still below expectations in the field. However, recently, an increasing number of studies have been focusing on the microscopic aspect after realizing the inevitable importance of this aspect in the technology of reactors.
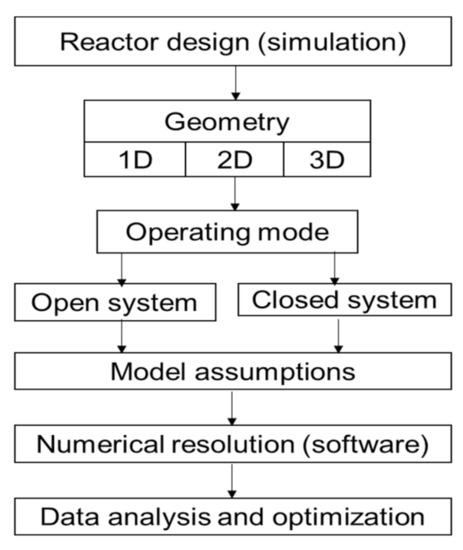
Several authors have worked on the modeling of physical phenomena in thermochemical energy storage reactors [27]. The simulation model used in this paper describes the decomposition of the material inside the reactor. The coupling of heat and mass transfer as well as kinetic equations has been implemented in the Comsol Multiphysics software using the infinite method, to analyze the physical phenomena involved in the reactor. A synthesis of those equations has been made in the following Table 1 to have a general dashboard and a schematic algorithm ( Figure 2 ) for reactor sizing.
An important aspect in the design of reactors is the design of the heat exchanger that must supply and remove heat before and after the reactions. This aspect also deserves special attention because the efficiency of the system depends on it as well. In addition, in the literature, many thermochemical heat storage projects compact and cylindrical heat exchangers are recommended or used because of the large exchange surface they offer. It should be noted that efficient heat exchanger performance requires small temperature differences throughout the heat exchanger to increase the heat transfer coefficient and thus increase the quality of heat exchange.
For specific applications, the heat exchangers involved must be dimensioned before any design. In many cases, the sizing is based on the log mean temperature difference. Thus, based on the application requirements, many criteria are integrated into the choice of the selected model, but the thermal power seems to be the most important in thermochemical heat storage, although, for a definitive choice, other parameters such as the pressure drop, the thermal mass, the encumbrance, or the clogging had to be considered. Heat exchanger sizing software such as COMSOL Multiphysics offers a high range of functionality for optimal sizing of the heat exchangers. Moreover, many recent works in thermochemical heat storage have used this software with very satisfactory results [28][29]. The innovative aspect of heat exchangers used in thermochemical heat storage is that most of them are heat exchangers involving a solid and a fluid. The tube containing the heat transfer fluid is in direct contact with the reactive bed, and the heat it contains is directly supplied mainly by conduction to the reactive bed during the loading phase.
It should be noted that one of the important aspects of thermochemical heat storage as mentioned above is the reactor and its optimization so that the energy density of the materials used may be efficiently exploited. Consequently, the recent investigations have been focused for the most part on the characterization of the reactor. The projects listed in Table 8 are very few of the projects in thermochemical heat storage, but the results obtained are very encouraging and deserve to be deepened and improved. More details on the projects carried out at the material and reactor level for TCHS can be found in the recent work carried out by Lin et al. [30].
The problem of mass and heat transfer has remained until now one of the principal barriers to thermochemical heat storage. To alleviate this problem, several techniques are used to get closer to a transitional solution or a compromise between several parameters. D. Aydin et al. [31] carried out a literature review in which they tried to list the methods of improving the heat and mass transfer addressed by some projects. These methods consist essentially of the use of composite materials, which consists of impregnating salt hydrates or other storage materials in porous materials by using different approaches. This technique enhances the rate of sorption of the storage materials that are trapped in the porous matrices, thus increasing the rate of the reaction and improving the mass transfer. Some methods involve the addition of fins to the reactive bed to increase the thermal conductivity of the reactive bed and thus increase the heat transfer. In the same direction, some methods use specific heat exchanger structures to increase the contact surface between the heat transfer fluid and the reactive bed, and some others use compacted reactive bed structures in direct contact with the heat exchanger in a specific structure.
Apart from this aspect of improving the quality of heat and mass transfer, efforts must be made to ensure that the heat is efficiently stored and used. N’Tsoukpoe et al. [32] have demonstrated that for thermochemical storage in buildings, during the charging phase, about two-thirds of the heat charged into the salt hydrates is lost as condensation heat, which is released into the environment. By using a cascade algorithm in the process of thermochemical heat storage, they have been able to improve the energy and exergy efficiencies of the process by establishing that the heat recovered during the charging period with the use of cascade is about 1.8 times higher than that of the same process without cascade. The same authors in a recent study on the review of long-term thermochemical heat storage systems for residential applications have shown that the volumetric densities of energy storage displayed by processes based on solid hydrates are prohibitive for the long-term heat storage applications [33]. As a result, an overestimation of the data is often observed. It would be more interesting if the simulation works exploited the parameters and data from their experimental studies with a certain level of precision.
Although it is the most promising of the TCHS processes with very high heat densities and temperature ranges, this storage method suffers from a lack of investigations and projects, especially in the field of heat and mass transfer, which reveal the main limitations of this process. Additionally, the complexity of the technologies used makes the systems very expensive and complex to maintain. Future works and approaches in this area can reflect on the approaches, combining with other sciences, namely computational fluid dynamics, heat, and mass transfer in solid and in porous media, to fill the gaps in this process. Furthermore, the optimization algorithms used in the simulation works should be clear enough to allow the implementation of the results in the real prototype scale.