
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tommaso Gori | + 809 word(s) | 809 | 2021-02-01 07:51:40 | | | |
| 2 | Camila Xu | Meta information modification | 809 | 2021-02-05 10:22:57 | | |
Video Upload Options
Restenosis is defined as the reduction in lumen size of an artery after intra-arterial intervention.
1. The Mechanisms of Restenosis
Restenosis is defined as the reduction in lumen size of an artery after intra-arterial intervention. While restenosis prior to the advent of coronary stents was mostly caused by recoil, in-stent restenosis is a response-to-injury process caused by formation of a tissue called neointima. For BMS, the theory postulated that this neointima is mostly composed of fibrotic tissue with a gradual and progressive onset over months, which led to the concept that in-stent restenosis is a benign phenomenon. In contrast, reports on the presentation of restenosis have consistently shown—without differences between BMS and DES—that more than 50% of the patients with in-stent restenosis present with unstable angina and myocardial infarction [1]. The initiating stimulus for restenosis is endothelial denudation and/or mechanical injury to the vessel wall, triggering an inflammatory response which can also be measured in terms of increase in circulatory markers such as c-reactive protein, amyloid A and fibrinogen [2][3]. Upon implantation, endothelial denudation and the exposure of components of the vascular extracellular matrix like collagen causes activation and adhesion of platelets. As well, the stent struts cause, proportionally to their thickness and dependent on the design of the stent, a disturbance in the flow that triggers the formation of large fibrin clots, further causing flow disturbances close to the struts and activating homeostatic reactions characterized by phagocyte invasion. In this context, the immunosuppressive action of the drug eluted might also have paradoxically negative effects, inhibiting tissue repair reaction and fibrin removal. These processes might explain the acute presentation of restenosis, as the activation of the thrombotic cascade ensuing from incomplete thrombin removal, although not causing occlusive thrombus, activates the recruitment of inflammatory cells such as monocytes, T cells, neutrophils via expression of adhesion molecules (ICAM, VCAM, intercellular, and vascular adhesion molecules), production of chemoattractant molecules (MCP-1 or monocyte chemoattractant protein-1, Interleukin (IL)-8) by endothelial, and smooth muscle cells and the production of growth factor such as PDGF (platelet-derived growth factor), bFGF (fibroblast growth factor), TGF (transforming growth factor)-beta, IGF (insulin-like growth factor), VEGF (vascular endothelial growth factor, and thrombin (Table 1, Figure 1).
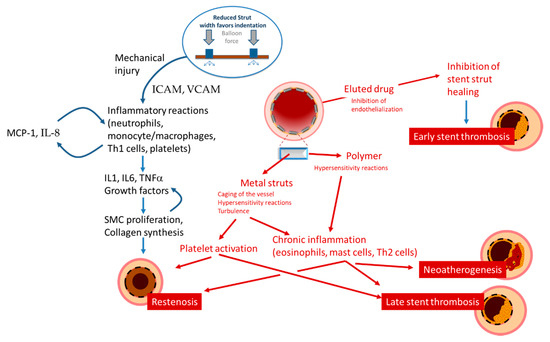
Figure 1. Schematic presentation of the mechanisms leading to restenosis, thrombosis, and neoatherosclerosis.
Table 1. Mechanisms and mediators involved in restenosis.
| Mechanism(s) Involved | Mediators and Signaling | |
|---|---|---|
| Response to Damage | ||
| Proliferation | Migration of smooth muscle cells, production of membrane metalloproteinases | Cytokines (IL-1, IL-6, TNF-alpha) Growth factors (PDGF, IGF, FGF, VEGF) |
| Remodeling | Remodeling of the neointima, deposition of extracellular matrix, neoatherogenesis | Macrophages/Foam cells Cytokines (IFNgamma) Growth factors (PDGF, TGFbeta, IGF, VEGF) |
| Thrombus Formation | Adhesion and activation of platelets Recruitment and diapedesis of Inflammatory cells |
Expression of vWF and TF Turbolent flow Nitric oxide, Thrombin Adhesion molecules Chemotactic factors (IL-8, MCP-1) Cytokines (IL-1, IL-6, TNF-alpha) Growth factors (PDGF, Thrombin) |
TNF: tumor necrosis factor; vWF: von Willebrand factor; IFN: interferon; FGF: fibroblast growth factor; all other abbreviations as in text.
The cytokines involved in these processes include IL-1, IL-6, and TNF-alpha [4]. This cascade is believed to activate a self-sustaining positive feedback loop of autocrine/paracrine messages that leads to stimulation of vascular smooth muscle cells growth and the production of extracellular matrix components. Matrix metalloproteinases activated by plasmin and their tissue inhibitors are also important mediators of this remodeling process. The formation of an endothelium is a critical step in these processes: an efficient endothelium may inhibit smooth muscle cell proliferation and neointimal growth; impaired neoendothelialization is associated with increased neointima proliferation [5]. However, studies have shown that stenting with BMS, and even more with of first generation DES, inhibits endothelialization [6][7]. Several lines of evidence also suggest that the above signaling cascades following coronary damage and stent implantation may also mobilize bone marrow-derived CD34-postive stem cells, which may then differentiate along endothelial or smooth muscle cell lines, in a balance between endothelial-like stem cell responses that favor reendothelialization and smooth muscle-like stem cell responses that promote restenosis [8].
2. Discussion
A number of potential therapies have attempted to address or reduce the incidence of restenosis beyond what already acheived with newer generation drug eluting stents. Based on the concept that oxidative stress may play an important regulatory role in inflammatory states (also supported by the fact that elevated systemic level of oxidative stress markers predict restenosis [9]), a number of trials have tested the effects of antioxidants on the incidence of restenosis. Probucol, a lipid-lowering agent with strong antioxidant properties, has shown to decrease the risk of restenosis and major adverse events by 41% and 31% in a meta-analysis of 15 studies and 859 patients, mainly treated with BMS or old-generation DES [10]. Probucol-eluting stents have also been tested, but they did not show superiority as compared to newer-generation DES in a large randomized trial [11]. The use of other drugs, such as sirolimus, colchicine, prednisolone and other reviewed in [12] has shown variable results and will need to be further investigated.
References
- Chen, M.S.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 2006, 151, 1260–1264, doi:10.1016/j.ahj.2005.08.011.
- Schnorbus, B.; Daiber, A.; Jurk, K.; Warnke, S.; Koenig, J.; Lackner, K.J.; Munzel, T.; Gori, T. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: A randomized, blinded, parallel study. Eur. Heart J. 2020, 41, 3144–3152, doi:10.1093/eurheartj/ehz917.
- Gori, T. Endothelial Function: A Short Guide for the Interventional Cardiologist. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19123838.
- Donners, M.M.; Daemen, M.J.; Cleutjens, K.B.; Heeneman, S. Inflammation and restenosis: Implications for therapy. Ann. Med. 2003, 35, 523–531, doi:10.1080/07853890310014876.
- Christen, T.; Verin, V.; Bochaton-Piallat, M.; Popowski, Y.; Ramaekers, F.; Debruyne, P.; Camenzind, E.; van Eys, G.; Gabbiani, G. Mechanisms of neointima formation and remodeling in the porcine coronary artery. Circulation 2001, 103, 882–888, doi:10.1161/01.cir.103.6.882.
- Puricel, S.; Kallinikou, Z.; Espinola, J.; Arroyo, D.; Goy, J.J.; Stauffer, J.C.; Baeriswyl, G.; Smits, P.C.; Cook, S.; Togni, M. Comparison of endothelium-dependent and -independent vasomotor response after abluminal biodegradable polymer biolimus-eluting stent and persistent polymer everolimus-eluting stent implantation (COMPARE-IT). Int. J. Cardiol. 2016, 202, 525–531, doi:10.1016/j.ijcard.2015.09.085.
- Togni, M.; Raber, L.; Cocchia, R.; Wenaweser, P.; Cook, S.; Windecker, S.; Meier, B.; Hess, O.M. Local vascular dysfunction after coronary paclitaxel-eluting stent implantation. Int. J. Cardiol. 2007, 120, 212–220, doi:10.1016/j.ijcard.2006.09.021.
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066, doi:10.1016/j.jcin.2011.05.025.
- Moldoveanu, E.; Mut-Vitcu, B.; Tanaseanu, G.R.; Marta, D.S.; Manea, G.; Kosaka, T.; Vidulescu, C.; Tanaseanu, C. Low basal levels of circulating adiponectin in patients undergoing coronary stenting predict in-stent restenosis, independently of basal levels of inflammatory markers: Lipoprotein associated phospholipase A2, and myeloperoxidase. Clin. Biochem. 2008, 41, 1429–1433, doi:10.1016/j.clinbiochem.2008.09.109.
- Liu, J.; Li, M.; Lu, H.; Qiao, W.; Xi, D.; Luo, T.; Xiong, H.; Guo, Z. Effects of probucol on restenosis after percutaneous coronary intervention: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0124021, doi:10.1371/journal.pone.0124021.
- Kufner, S.; Sorges, J.; Mehilli, J.; Cassese, S.; Repp, J.; Wiebe, J.; Lohaus, R.; Lahmann, A.; Rheude, T.; Ibrahim, T.; et al. Randomized Trial of Polymer-Free Sirolimus- and Probucol-Eluting Stents Versus Durable Polymer Zotarolimus-Eluting Stents: 5-Year Results of the ISAR-TEST-5 Trial. JACC Cardiovasc. Interv. 2016, 9, 784–792, doi:10.1016/j.jcin.2016.01.009.
- Guerra, E.; Byrne, R.A.; Kastrati, A. Pharmacological inhibition of coronary restenosis: Systemic and local approaches. Expert Opin. Pharmacother. 2014, 15, 2155–2171, doi:10.1517/14656566.2014.948844.




