
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arthur Kopylov | + 2594 word(s) | 2594 | 2021-05-19 06:29:07 | | | |
| 2 | Karina Chen | Meta information modification | 2594 | 2021-05-24 04:52:45 | | | | |
| 3 | Karina Chen | Meta information modification | 2594 | 2021-05-24 05:23:26 | | |
Video Upload Options
Plant sterols are inherent compounds of many nutritional supplements and food additives. Sterols are chemical compounds based on 1,2-cyclopentaneperhydrophenantrene and are characterized by hydroxyl moiety at the 3C position and the side chain at the 17C position. The latter makes them structurally similar to pregnenolone, which is a fundamental molecule for all 17-ketosteroids generation. The major natural source of dietary plant sterols are vegetables, fruits, berries, and vegetable oil.
1. The Prevalence and Variety of Plant Sterols
The minimal intake of plant sterols per day should be at least 1 g to produce an appreciable cholesterol-lowering effect [1]. However, fruits, vegetables, and plant oils are hard to be considered as natural sources of plant sterols due to the insufficient content of these compounds. For example, the content of naturally occurring sterols in fruits and vegetables is ranged between 38 to 439 mg/kg of fresh weight, whereas in grains, their content reaches up to 1780 mg/kg [1][2]. Thus, about 2 kg of fruits and vegetables or about 1 kg of grains has to be consumed daily to obtain the necessary 1 g of plant sterols. Other nutrition experts argue that 200 to 400 mg of plant sterols per day is the optimal dose for handling diet and being processed by the gastrointestinal tract [3]. A typical western diet contains about 300 mg of plant sterols in daily intake [4] and, accordingly, other sources including corn fiber oil, soy, rapeseeds, or rice bran oil are considered as the alternative feedstock of plant sterols because they contain up to 15% of plant sterols [1][5].
About 300 various species of plant sterols are described [6][7][8], which differed by side chain at C24 position and can be specific for certain plant species. This variety of compounds covers free sterols and their ester conjugates with fatty acids at the 3β-OH moiety, acylated sterol glycosides, and sterol glycosides (typically with glucose), which are specifically absent in all animals. The most abundant and typical plant sterols are represented by campesterol and stigmasterol. The main plant enzymes responsible for maintaining the balance of different sterols and their biosynthesis are 3-hydroxy-3-methylglutaryl-CoA reductase, C24-sterol methyltransferase, and C22-sterol desaturase. A human can discriminate non-cholesterol plant sterols and excrete most of them with feces together with bile acids, which suggests the existence of a mechanism responsible for retaining endogenous cholesterol over plant sterols [9].
2. Factor Associated with the Efficacy of Plant Sterols Absorption
The clinical efficacy of plant sterols is predominantly associated with their cholesterol-lowering effect. Numerous recent studies were dedicated to important aspects (chemical form of sterols and supplements, food matrix, way of delivery, and frequency of intake) influencing sterols absorption and their action efficacy [10][11][12].
No significant difference in plant sterol efficacy was established when supplemented with low-fat or high-fat foods [13] as wells as no difference being found between the consumption of plant sterols or stanols. The cholesterol-lowering effect for both sterols and stanols was estimated as 0.34 mmol/L if the consumed mean daily dose was 2.15 g. However, an apparent tendency (p = 0.054) of lower efficacy was established for single vice multiple intakes of plant sterol supplements. Other research groups found that the intake of different dietary plant sterols leads to a comparable decrease in plasma cholesterol, but, in contrast, stanols are up to 50-fold less effective [14][15].
The use of food supplements with plant sterols together with the lipid-lowering therapy demonstrated an additive effect. When atorvastatin and plant sterols were used combined, the lowering effect of total cholesterol and LDL-C was estimated at 22% and 38%, respectively (p < 0.05). In contrast, the effect of monotherapy by the lipid-lowering agent in a dose of 40 mg was 3% and 22% for total cholesterol and LDL-C, respectively. Notwithstanding, some epidemiological studies reported the increased risk of cardiovascular events and the net negative effect if the plasma level of plant sterols reaches the upper normal [7][16].
A meta-analysis of a large number of studies (n = 124 studies in total) established the difference in plant sterols absorption efficacy depending on the type of food matrix [17]. The effect of plant sterols and their absorption was more pronounced when consumed with drinking food (9.5% lowering of cholesterol, p < 0.001) rather than with solid food. Moreover, the effect was more explicit when sterols are consumed at lunch instead of breakfast. Although it seems on the surface that the high-fat food matrix is the most appropriate carrier for sterols absorption, low-fat may also be competent if products contain emulsifiers [18]. Therefore, the total cholesterol-lowering effect and rate of sterols absorption were irrespective of total fat content in the meal [19]. Nevertheless, a robust dose-dependent response was observed when sterols and stanols were analyzed separately. No significant difference was detected between the efficacy of free and esterified sterols (less than 2% in a dose between 0.6 and 3.3 g per day), thus plant sterols and non-esterified stanols contribute almost equally in cholesterol-lowering effect [14][20].
Since many food supplements and additives with plant sterols are currently manufactured in the form of capsules or pills, this raises the question about their effectiveness compared to the regular intake. The meta-analysis carried in the 2013 year and covered eight eligible clinical trials showed a similar effect consisting of 0.31 mmol/L (p < 0.0001) cholesterol-lowering for both dietary sterols and sterols delivered in capsules or pills [21][22]. However, it should be warned that the majority of studies lack information about particle size, solubility, and bioavailability, which are all critical characteristics when discussing capsule and pill formulation as possible carrier agents [23][24][25]. Different studies indicated that an excretion rate of sterols and cholesterol is the most critical factor affecting intestinal absorption of plant sterols [26][27][28]. The excretion rate is determined by ATP-binding cassette sub-family G member 5/8 transporter (ABCG5/ABCG8) and only brain cells are the exclusion of this rule (Figure 1).
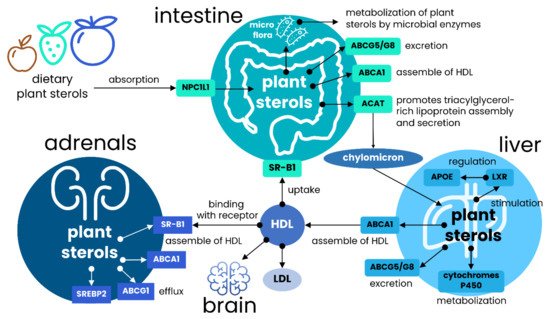
Figure 1. General scheme of the major routes of plant sterols in human. Plant sterols and dietary cholesterol are mainly absorbed in the intestine, and Niemann-Pick C1-like 1 (NPC1L1) transporter plays a vital role in the regulation of initial absorption. After absorption, sterols are esterified in support with ACAT and transported to the liver following the incorporation into chylomicrons. In contrast, unesterified sterols are pumped out by ABCG5/ABCG8 transporters, which are the major cholesterol and plant sterols transporters. The plasma efflux of both plant sterols and cholesterol is regulated by ABCA1, which is involved in the assembling of HDL-like particles with the assimilated sterols. This transporter is also critically important in plant sterols and cholesterol efflux after delivering chylomicrons from enterocytes to liver cells. Plant sterols are capable of stimulating LXR receptors regulating APOE expression, which is essential for HDL and LDL assembly and uptake; and can be partially catalyzed by microsomal cytochromes. Binding with LXR receptors upregulates ABCG5/G8 transporters and thus enhances cholesterol and plants sterols absorption. The exported HDL-like particles with the incorporated plant sterols are trapped by SR-IB receptors expressed on the liver and adrenal glands cell surface. This receptor plays a pivotal role in the uptake of cholesterol through HDL and, most importantly, for brain lipids metabolism, where HDL is the primary source of lipids and cholesterol uptake.
Liver X receptor (LXR) is the primary positive regulator of these transporters, and mutations in either of these genes cause the condition of sitosterolemia, characterized by the accumulation of cholesterol and plant sterols in plasma and tissues [29]. It has been found that only up to 2% of plant sterols enter the system, whereas the rest is pumped into the bile [30]. At least in animal models with the deleted Abcg5 and Abcg8, the increase of dietary plant sterols absorption in 2–3-fold changes and associated 30-folds increase in plasma cholesterol has been demonstrated [31]. Alternatively, it was reported that murine deficiency in ABCG5/ABCG8 demonstrated the level of plasma and liver sterols comparable with the wild-type mice; however, the level of non-cholesterol sterols was elevated up to 30-folds, indicating that sterols are preferentially secreted into bile. Besides, some authors revealed that such a population of knockout animals is characterized by the decreased mRNAs level for 13 enzymes involved in cholesterol biosynthesis [31][32].
Assumingly, disruption of even the Abcg5 gene alone is already enough to increase meaningfully the rate of dietary sterols absorption, while maintaining the rate of cholesterol secretion steady. The genome-wide associated study provided by three European biobanks (Iceland, Denmark, and the UK) confirmed that nine rare polymorphisms in Abcg5/8 loci are tightly associated with the rate of plant sterols absorption, their excretion rate, level of LDL, and the risk of coronary artery disease [33].
The affinity for and the solubility in bile salt micelles born completely different opinions regarding the factors affecting absorption of plant sterols. The suggestion came from the upshot of experiments on human colorectal adenocarcinoma cells (Caco-2), when intestinal absorption of cholesterol was inhibited because of plant sterols being well-solubilized in bile salts and, thence, they competitively limit the solubilizing capacity for cholesterol [34].
3. Health Improvement Properties of Plant Sterols
Plants sterols are believed to be a necessary compound of the human diet and recommended to add as a food supplement or functional food. In the early 1950s, it was discovered and clinically approved cardioprotective effect of plants sterol β-sitosterol caused by its cholesterol-lowering property mediated by the replacement of cholesterol in HDL/LDL particles [35]. Years later, many studies showed that consuming even 2.5 g of plant sterols in a daily diet is enough to decrease the level of circulating triglycerides by 9.5%-13% [17][18]. Plants sterols make a significant impact on the central nervous system. Due to the presence of the alkyl moiety at the C24 position, plant sterols cannot be converted into more polar molecules, unlike endogenous cholesterol. Therefore, sterols can be irreversibly accumulated and integrated into the cell membrane after entering into the brain circulation [36].
The exact role of dietary plant sterols is not yet recognized; thus, it cannot be judged as strictly positive or detrimental. Long-term exposure to plant sterols leads to an increased level of sterols in brain cells and sufficiently reduces the density of amyloid plaques in patients with Alzheimer’s disease, but does not lead to the improvement of cognitive function and memory [37]. Since the early stage of Alzheimer’s disease is associated with the impairment of BBB functioning, the reduced concentration of plant sterols in cerebrospinal fluid (CSF) was supposed as a promising predictive biomarker with an 85% sensitivity and 75% specificity [38][39]. Application of plant sterols for the management of neurodegenerative diseases therapy was proposed due to their immune-modulating activity engaged in the augmentation of bone-marrow-derived macrophages polarization to anti-inflammatory phenotype caused by the depletion of IL-10α (p < 0.01) but the sustained elevation of interleukins IL-1β, IL-6, and IL-12 in 16 days after treatment [40][41]. Recent findings demonstrate the ability of plant sterols to restore the impaired uptake of cholesterol also in patients with multiple sclerosis, when plant sterols additives, at least in the animal models, may stimulate the re-myelination process [42]. Sterols extract from Aloe vera stimulates the expression of Fatp1, Acox1, and Cpt1 genes in a dose-dependent manner through the activation of PPAR receptors, which produce a sufficient antioxidant response resulting in the increased level of glutathione and the diminished expression of IL-18 supporting the recovery and amelioration of patients with neurodegenerative diseases [43][44].
Skin regeneration and wound healing are currently the main points, where dietary sterols are widely utilized. The regenerative property of plant sterols is mediated in the same manner as the anti-inflammatory effect briefly described above. Ethanolic extract of traditional Thai medical plant parts (seeds, root, and pericarp) obtained from Garcinia mangostana L., Glycyrrhiza glabra L., Nigella sativa L, exhibited an inhibiting property toward superoxide dismutase (SOD) and nitric oxide (NO), while the extracts from G. mangostana and G. glabra at a concentration of 5 µg/mL can enhance cells proliferative activity supporting the positive effect of plant sterols on wound regeneration and recovery after damage [45]. Animals who were given a dermal wound and further treated on topical application with brassinosteroid for 10 days demonstrated almost completely suppressed expression of tumor necrosis factor-α (TNF-α) transforming growth factor-β (TGF-β) indicating the enhanced proliferative activity and stimulation of cells migration caused by PI3K/Akt (phosphoinositide 3-kinases/protein kinase B) signaling pathway [46].
The potential anabolic properties of plants are very attractive in professional sport. Considering the growing offers of plant-based food supplements and additives intended for the intentional improvement of elite athlete abilities, WADA (World Anti-Doping Agency) included some suspected dietary sterols in the monitoring list. Evidence exists that the anabolic effect of some of the sterols (for example, 20β-OH-ecdysone) is caused by the binding with estrogen receptors, thus, such sterols can be even more effective in comparison to some synthetic steroids [47]. There is an accumulating number of reports about the targeted effect of 20β-OH-ecdysone on muscle cell size and elevation of a myonuclear number of the regenerating myocytes, which are very convincing in anabolic and modulating properties of this sterol, albeit mediated in an unknown way [48][49]. The main metabolic product, poststerone, also fosters the increase of muscle type fiber cross-sectional area but to a relatively lower degree compared to its parental compound [50]. Eventually, based on the body tissue analysis (muscle cells growth, an increase of fibers cross-sectional area) and known aspects of the protein synthesis gain, most authors proposed that benefits of 20β-OH-ecdysone are caused by the activation of the PI3K/Akt pathway. Due to possible androgenic and growth-stimulating effects, 20β-OH-ecdysone has been examined on the subject of acceleration of bone mass augmentation. Although the bone mass augmentation is pre-determined by gender-specific hormonal background and, specifically, by estrogen receptor loading, it has been demonstrated that 20β-OH-ecdysone provides equal impact regardless of the gender feature [51][52][53]. The possible implication of estradiol receptors rather than androgen receptor activation has been previously proposed based on the in silico molecular docking [54]. However, the molecular model contradicts the results of the experiment, displaying that the regulatory activity of plant sterols bypasses this way of interaction, and they do not bind with estradiol receptors [53].
The presence and origination of anabolic steroids (in particular, boldione) have been demonstrated in a tested group of volunteers who were administrated with plant sterols, however, no significant correlation was established between the excretion rate of 17-ketosteroids precursors (for example, androstenediol) and the consumed amount or the frequency of plant sterols intake [55]. Before, it has been reported an in vitro transformation of the selected plant sterols into androgenic products (testosterone and androstenedione), which has been explained by the activity of residential gastrointestinal microflora [56][57][58]. Some plant sterols (sitosterol, campesterol, stigmasterol, episterol) can be utilized as precursors for the endogenous synthesis of human 17-ketosteroids, including DHEA (dehydroepiandrosterone) [59][60][61]. The occurrence of anabolic steroids in plants was rigorously reviewed by [62], who documented that the prevalence of progesterone (up to 80 ng/g) was in more than 80%, and the prevalence of androgens (androsterone up to 11.4 ng/g, testosterone up to 80 ng/g, and epitestosterone up to 110 ng/g) was in more than 70%, and 17β-estradiol (in amounts up to 40 pg/g) was in nearly in 50% cases among 128 tested plant species of 50 families. Most of the detected and reported steroids were reported as not being contaminants of plant subjects, but instead, they were substrate-specific intermediates that naturally occur in plants. Detection of androstadienone and androstenedione at a concentration of 2.20 ng/g has been reported for Nicotiana tabacum [63], pine pollen [64], and even in potato, where marginal concentration did not exceed 50 pg/g [65].
4. Conclusion
So far, the exact effect of plant sterols is poorly predictable and requires a deep investigation of nutrigenomic, epigenetic, and even ecological factors before plan and develop diet recommendations purposed to improve abilities in sport or avoid cardiovascular complications, or retard skin aging, etc. Consequently, a very careful personalized approach based on the highly-specific bioactive compound, including a specific sterol, should be developed, while considering different nutritional needs and different categories of the population.
References
- Moreau, R.A. Composition of Plant Sterols and Stanols in Supplemented Food Products. J. AOAC Int. 2015, 98, 685–690.
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J. Phytosterol Contents of Edible Oils and Their Contributions to Estimat-ed Phytosterol Intake in the Chinese Diet. Foods 2019, 8, 334.
- Patel, S.B. Plant sterols and stanols: Their role in health and disease. J. Clin. Lipidol. 2008, 2, S11–S19.
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482.
- Cusack, L.K.; Fernandez, M.L.; Volek, J.S. The Food Matrix and Sterol Characteristics Affect the Plasma Cholesterol Lowering of Phytosterol/Phytostanol. Adv. Nutr. 2013, 4, 633–643.
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochemistry 2016, 81, 819–834.
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37.
- Benveniste, P. Biosynthesis and Accumulation of Sterols. Annu. Rev. Plant Biol. 2004, 55, 429–457.
- Patel, S.B.; Honda, A.; Salen, G. Sitosterolemia: Exclusion of genes involved in reduced cholesterol biosynthesis. J. Lipid Res. 1998, 39, 1055–1061.
- Malina, D.M.T.; Fonseca, F.A.; Barbosa, S.A.; Kasmas, S.H.; Machado, V.A.; França, C.N. Additive effects of plant sterols supple-mentation in addition to different lipid-lowering regimens. J. Clin. Lipidol. 2015, 9, 542–552.
- Rysz, J.; Franczyk, B.; Olszewski, R.; Banach, M.; Gluba-Brzozka, A. The Use of Plant Sterols and Stanols as Lipid-Lowering Agents in Cardiovascular Disease. Curr. Pharm. Des. 2017, 23, 2488–2495.
- Sahebkar, A.; Serban, M.-C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192.
- Demonty, I.; Ras, R.T.; van der Knaap, H.C.M.; Duchateau, G.S.M.J.E.; Meijer, L.; Zock, P.L. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 2009, 139, 271–284.
- von Bergmann, K.; Sudhop, T.; Lütjohann, D. Cholesterol and plant sterol absorption: Recent insights. Am. J. Cardiol. 2005, 96, 10D–14D.
- Kreuzer, J. Phytosterols and phytostanols: Is it time to rethink that supplemented margarine? Cardiovasc. Res. 2011, 90, 397–398.
- Silbernagel, G.; Baumgartner, I.; März, W. Cardiovascular Safety of Plant Sterol and Stanol Consumption. J. AOAC Int. 2015, 98, 739–741.
- Ras, R.T.; Geleijnse, J.M.; Trautwein, E.A. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose rang-es: A meta-analysis of randomised controlled studies. Br. J. Nutr. 2014, 112, 214–219.
- Thomsen, A.B.; Hansen, H.B.; Christiansen, C.; Green, H.; Berger, A. Effect of free plant sterols in low-fat milk on serum lipid profile in hypercholesterolemic subjects. Eur. J. Clin. Nutr. 2004, 58, 860–870.
- Doornbos, A.M.E.; Meynen, E.M.; Duchateau, G.S.M.J.E.; Van Der Knaap, H.C.M.; A Trautwein, E. Intake occasion affects the serum cholesterol lowering of a plant sterol-enriched single-dose yoghurt drink in mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2006, 60, 325–333.
- Nestel, P.; Cehun, M.; Pomeroy, S.; Abbey, M.; Weldon, G. Cholesterol-lowering effects of plant sterol esters and non-esterified stanols in margarine, butter and low-fat foods. Eur. J. Clin. Nutr. 2001, 55, 1084–1090.
- Amir Shaghaghi, M.; Abumweis, S.S.; Jones, P.J.H. Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: Results of a systematic review and meta-analysis. J Acad. Nutr. Diet. 2013, 113, 1494–1503.
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981.
- Maki, K.C.; Lawless, A.L.; Reeves, M.S.; Dicklin, M.R.; Jenks, B.H.; Shneyvas, E.; Brooks, J.R. Lipid-altering effects of a dietary supplement tablet containing free plant sterols and stanols in men and women with primary hypercholesterolaemia: A randomized, placebo-controlled crossover trial. Int. J. Food Sci. Nutr. 2012, 63, 476–482.
- McPherson, T.B.; Ostlund, R.E.; Goldberg, A.C.; Bateman, J.H.; Schimmoeller, L.; Spilburg, C.A. Phytostanol tablets reduce human LDL-cholesterol. J. Pharm. Pharmacol. 2010, 57, 889–896.
- Ottestad, I.; Ose, L.; Wennersberg, M.H.; Granlund, L.; Kirkhus, B.; Retterstøl, K. Phytosterol capsules and serum cholesterol in hy-percholesterolemia: A randomized controlled trial. Atherosclerosis 2013, 228, 421–425.
- Plösch, T.; Bloks, V.W.; Terasawa, Y.; Berdy, S.; Siegler, K.; van der Sluijs, F.; Kema, I.P.; Groen, A.K.; Shan, B.; Kuipers, F.; et al. Sitosterolemia in ABC-Transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 2004, 126, 290–300.
- Yu, X.-H.; Qian, K.; Jiang, N.; Zheng, X.-L.; Cayabyab, F.S.; Tang, C.-K. ABCG5/ABCG8 in cholesterol excretion and atherosclerosis. Clin. Chim. Acta 2014, 428, 82–88.
- Calpe-Berdiel, L.; Escolà-Gil, J.C.; Blanco-Vaca, F. New insights into the molecular actions of plant sterols and stanols in choles-terol metabolism. Atherosclerosis 2009, 203, 18–31.
- Wouters, E.; De Wit, N.M.; Vanmol, J.; Van Der Pol, S.M.A.; Hof, B.V.H.; Sommer, D.; Loix, M.; Geerts, D.; Gustafsson, J.A.; Steffensen, K.R.; et al. Liver X Receptor Alpha Is Important in Maintaining Blood-Brain Barrier Function. Front. Immunol. 2019, 10, 1811.
- Ostlund, R.E.J.; McGill, J.B.; Zeng, C.-M.; Covey, D.F.; Stearns, J.; Stenson, W.F. Gastrointestinal absorption and plasma kinetics of soy Delta(5)-phytosterols and phytostanols in humans. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E911–E916.
- Yu, L.; Hammer, R.E.; Li-Hawkins, J.; von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA 2002, 99, 16237–16242.
- Yu, L.; von Bergmann, K.; Lutjohann, D.; Hobbs, H.H.; Cohen, J.C. Selective sterol accumulation in ABCG5/ABCG8-deficient mice. J. Lipid Res. 2004, 45, 301–307.
- Helgadottir, A.; Thorleifsson, G.; Alexandersson, K.F.; Tragante, V.; Thorsteinsdottir, M.; Eiriksson, F.F.; Gretarsdottir, S.; Björnsson, E.; Magnusson, O.; Sveinbjornsson, G.; et al. Genetic variability in the absorption of dietary sterols affects the risk of coronary artery disease. Eur. Heart J. 2020, 41, 2618–2628.
- Ikeda, I. Factors Affecting Intestinal Absorption of Cholesterol and Plant Sterols and Stanols. J. Oleo Sci. 2015, 64, 9–18.
- Best, M.M.; Duncan, C.H.; Van Loon, E.J.; Wathen, J.D. Lowering of serum cholesterol by the administration of a plant sterol. Circulation 1954, 10, 201–206.
- Wang, Y.; Muneton, S.; Sjövall, J.; Jovanovic, J.N.; Griffiths, W.J. The Effect of 24S-Hydroxycholesterol on Cholesterol Homeostasis in Neurons: Quantitative Changes to the Cortical Neuron Proteome. J. Proteome Res. 2008, 7, 1606–1614.
- Vanmierlo, T.; Popp, J.; Kölsch, H.; Friedrichs, S.; Jessen, F.; Stoffel-Wagner, B.; Bertsch, T.; Hartmann, T.; Maier, W.; Von Bergmann, K.; et al. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr. Scand. 2011, 124, 184–192.
- Lobo, A.; Quintanilla, M.A. The search of new biomarkers to identify Alzheimer’s disease: An editorial comment to T. Vanmierlo et al. ‘The Plant Sterol Brassicasterol and Additional CFS Biomarker in Alzheimer’s Disease ‘(1). Acta Psychiatr. Scand. 2011, 124, 163–164.
- Vanmierlo, T.; Rutten, K.; Friedrichs, S.; Bloks, V.W.; Blokland, A. Cerebral accumulation of dietary derivable plant sterols does not interfere with memory and anxiety related behavior in Abcg5−/− mice. Plant Foods Hum. Nutr. 2011, 66, 149–156.
- Liu, R.; Hao, D.; Xu, W.; Li, J.; Li, X.; Shen, D.; Sheng, K.; Zhao, L.; Xu, W.; Gao, Z.; et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm. Biol. 2019, 57, 161–168.
- Valerio, M.; Liu, H.-B.; Heffner, R.; Zivadinov, R.; Ramanathan, M.; Weinstock-Guttman, B.; Awad, A.B. Phytosterols ameliorate clinical manifestations and inflammation in experimental autoimmune encephalomyelitis. Inflamm. Res. 2010, 60, 457–465.
- Saher, G.; Rudolphi, F.; Corthals, K.; Ruhwedel, T.; Schmidt, K.-F.; Löwel, S.; Dibaj, P.; Barrette, B.; Möbius, W.; Nave, K.-A. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat. Med. 2012, 18, 1130–1135.
- Nomaguchi, K.; Tanaka, M.; Misawa, E.; Yamada, M.; Toida, T.; Iwatsuki, K.; Goto, T.; Kawada, T. Aloe vera phytosterols act as ligands for PPAR and improve the expression levels of PPAR target genes in the livers of mice with diet-induced obesity. Obes. Res. Clin. Pract. 2011, 5, e190–e201.
- Klaikeaw, N.; Wongphoom, J.; Werawatganon, D.; Chayanupatkul, M.; Siriviriyakul, P. Anti-inflammatory and anti-oxidant effects of Aloe vera in rats with non-alcoholic steatohepatitis. World J. Hepatol. 2020, 12, 363–377.
- Siriwattanasatorn, M.; Itharat, A.; Thongdeeying, P.; Ooraikul, B. In Vitro Wound Healing Activities of Three Most Commonly Used Thai Medicinal Plants and Their Three Markers. Evid. Based Complement. Altern. Med. 2020, 2020, 1–11.
- Wouters, E.; De Wit, N.M.; Vanmol, J.; Van Der Pol, S.M.A.; Hof, B.V.H.; Sommer, D.; Loix, M.; Geerts, D.; Gustafsson, J.A.; Steffensen, K.R.; et al. Liver X Receptor Alpha Is Important in Maintaining Blood-Brain Barrier Function. Front. Immunol. 2019, 10, 1811.
- Parr, M.K.; Botrè, F.; Naß, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport 2015, 32, 169–173.
- Tóth, N.; Szabó, A.; Kacsala, P.; Héger, J.; Zádor, E. 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine 2008, 15, 691–698.
- Bathori, M.; Toth, N.; Hunyadi, A.; Marki, A.; Zador, E. Phytoecdysteroids and Anabolic-Androgenic Steroids—Structure and Effects on Humans. Curr. Med. Chem. 2008, 15, 75–91.
- Csábi, J.; Rafai, T.; Hunyadi, A.; Zádor, E. Poststerone increases muscle fibre size partly similar to its metabolically parent compound, 20-hydroxyecdysone. Fitoterapia 2019, 134, 459–464.
- Dai, W.; Zhang, H.; Zhong, Z.A.; Jiang, L.; Chen, H.; Lay, Y.-A.E.; Kot, A.; Ritchie, R.O.; Lane, N.E.; Yao, W. β-Ecdysone Augments Peak Bone Mass in Mice of Both Sexes. Clin. Orthop. Relat. Res. 2015, 473, 2495–2504.
- Kapur, P.; Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Beneficial effects of β-Ecdysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomedicine 2010, 17, 350–355.
- Seidlova-Wuttke, D.; Christel, D.; Kapur, P.; Nguyen, B.T.; Jarry, H.; Wuttke, W. Beta-ecdysone has bone protective but no estro-genic effects in ovariectomized rats. Phytomedicine 2010, 17, 884–889.
- Parr, M.K.; Botrè, F.; Naß, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport 2015, 32, 169–173.
- Verheyden, K.; Noppe, H.; Vanhaecke, L.; Wille, K.; Bussche JVanden Bekaert, K. Excretion of endogenous boldione in hu-man urine: Influence of phytosterol consumption. J. Steroid. Biochem. Mol. Biol. 2009, 117, 8–14.
- Kicman, A.T.; Gower, D.B. Anabolic steroids in sport: Biochemical, clinical and analytical perspectives. Ann. Clin. Biochem. Int. J. Lab. Med. 2003, 40, 321–356.
- Mareck, U.; Geyer, H.; Opfermann, G.; Thevis, M.; Schänzer, W. Factors influencing the steroid profile in doping control analysis. J. Mass Spectrom. 2008, 43, 877–891.
- Rozner, S.; Garti, N. The activity and absorption relationship of cholesterol and phytosterols. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 282–283, 435–456.
- Prager, N.; Bickett, K.; French, N.; Marcovici, G. A randomized, double-blind, placebo-controlled trial to determine the effective-ness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J. Altern. Complementary Med. 2002, 8, 143–152.
- Bain, B.J.; Chakravorty, S. Phytosterolemia. Am. J. Hematol. 2016, 91, 643.
- Mignarri, A.; Magni, A.; Del Puppo, M.; Gallus, G.N.; Björkhem, I.; Federico, A. Evaluation of cholesterol metabolism in cere-brotendinous xanthomatosis. J. Inherit. Metab. Dis. 2016, 39, 75–83.
- Janeczko, A.; Skoczowski, A. Mammalian sex hormones in plants. Folia Histochem. Cytobiol. 2005, 43, 71–79.
- Simerský, R.; Novák, O.; Morris, D.A.; Pouzar, V.; Strnad, M. Identification and Quantification of Several Mammalian Steroid Hormones in Plants by UPLC-MS/MS. J. Plant Growth Regul. 2009, 28, 125–136.
- Šaden-Krehula, M.; Tajić, M.; Kolbah, D. Testosterone, epitestosterone and androstenedione in the pollen of scotch pine P. silvestris L. Cell. Mol. Life Sci. 1971, 27, 108–109.
- Hartmann, S.; Lacorn, M.; Steinhart, H. Natural occurrence of steroid hormones in food. Food Chem. 1998, 62, 7–20.




