
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vilia Darma Paramita | + 5142 word(s) | 5142 | 2020-04-16 05:55:23 | | | |
| 2 | Rita Xu | -1745 word(s) | 3397 | 2020-04-18 14:44:07 | | | | |
| 3 | Rita Xu | -60 word(s) | 3337 | 2020-10-29 09:19:06 | | |
Video Upload Options
In the food industry, proteins are regarded as multifunctional systems whose bioactive hetero-polymeric properties are affected by physicochemical interactions with the surrounding components in formulations. Due to their nutritional value, plant proteins are increasingly considered by the new product developer to provide three-dimensional assemblies of required structure, texture, solubility and interfacial/bulk stability with physical, chemical or enzymatic treatment. This molecular flexibility allows them to form systems for the preservation of fresh food, retention of good nutrition and interaction with a range of microconstituents. While, animal- and milk-based proteins have been widely discussed in the literature, the role of plant proteins in the development of functional foods with enhanced nutritional profile and targeted physiological effects can be further explored. Molecular functionality of plant proteins in relation to their structural transformation i.e. gelation, phase separation and vitrification, is discussed in this review.
1. Structural Functionality of Plant Proteins from Low to High Solid Systems
1.1. Effect of Heating
Heating is the main physical factor to induce gelation in globular proteins, thereby, altering their functional properties dramatically. Upon heating, native globular molecules in aqueous solution unfold and expose the hydrophobic core facilitating polypeptide-polypeptide and polypeptide-water interactions [1]. This process is initiated by diminishing stability of hydrogen bonds in the hydration shell of the protein, which enables the rearrangement of chain segments to balance a multitude of attractive and repulsive forces [2]. At high enough concentrations, gels are formed upon cooling via disulfide, ionic, hydrophobic and hydrogen bonds [3].
Plant globular proteins, extracted from legume seeds, exhibit structure formation as for the animal counterparts with some variation according to their physicochemical fingerprints [4]. Thus, the β-sheet content of globulins in soy, kidney bean and field pea protein isolates relates positively to the small deformation properties of firmness and yield strength [5][6]. Large deformation properties depend primarily on the number of disulfide bridges. For example, the 2S fraction of soy protein produced flexible structures with reduced water-holding capacity than the 7S fraction due to less disulfide bridges [7]. The high content of disulfide bridges in native 11S fraction of lupin protein isolates prevented molecular reconfigurations upon moderate heat treatment [8]. At the isoelectric point (pH 4–5), aggregation of globulins extracted from soy, quinoa and sesame occurred since the ionization of amino-acid side chains is reduced. Heating can disrupt the disulfide bridges and in alkaline conditions, protein solubility is encouraged due to increased electrostatic repulsion [9][10][11].
Plant protein blends, with other biopolymers, are of interest in novel product development and in understanding the thermodynamic or kinetic processes, as a function of extrinsic factors, including temperature, pH and ionic strength, facilitates structural and functional manipulation. In a relatively dilute aqueous solution, plant proteins show co-gelation with animal proteins [12] or independent gelation that traces the structural characteristics of each component in the mixture [13]. This was shown in the synergistic enhancement of storage modulus, gel hardness and paste viscosity for a co-gelated 10–22% (w/v) pea protein with whey protein system [12] and phase separated 10–24% (w/v) soy protein isolate with gelatine system [13]. Gelation of two molecular fractions of soy protein, 11S and 2S in acidic conditions, showed that the high molecular weight fraction formed the continue phase supporting the discontinuous inclusions of the 2S fraction. Increasing concentrations of the latter were able to change dramatically the gelation temperature, network strength of the composite and the distribution of solvent between the two polymeric phases [14]. Heating soy or pea protein in mixture with micellar casein, in the presence of calcium ions, resulted in the formation of cohesive structures due to the “beneficial” distribution of the counterion within the two proteinaceous phases of the mixture [14].
Increasing additions of globular protein in processed food formulations creates condensed systems where the structural performance is primarily governed by the property of water molecules as plasticizers, as opposed to solvent in low-solid preparations [1][5]. In the case of soy glycinin (11S), a total solids content between 10 and 70% (w/w) retained the secondary structure of the polymer, which was mainly β-sheet followed by random coil, β-turn and α-helix. As expected, higher protein concentrations entrapped efficiently water molecules leading to the formation of firm gels [5]. At 80% (w/w) solids, soy glycinin exhibits mechanical properties of an amorphous network characterised by low molecular mobility with high strength and brittleness. These mechanical properties are reminiscent of the viscoelasticity of synthetic amorphous polymers and can be treated as such.
Within a certain temperature range (mainly at subzero temperatures) the condensed protein system (80% solids) records high values of shear storage modulus expected for a glassy consistency. In this supercooled region, there is limited molecular mobility of the amino acid backbone and side chains. Structural relaxation of the condensed matrix with heating generates free volume within the entangled polymer chains, with the material undergoing a glass transition indicated by the so-called “glass transition temperature, Tg”. On further heating, the mechanical properties of the high-solid glycinin matrix exhibit a rubbery consistency and the material softens progressively. Vitrification phenomena of condensed glycinin systems follow qualitatively the transition from the glassy to the rubbery state of high-solid globular proteins from animal sources. This allows comparisons to be made of the structural functionality for partial/total replacement of meat proteins in high-solid formulations [1][5].
1.2. Effect of High Pressure
High pressure is the most popular non-thermal treatment in the food industry for the modification of the structural properties of materials, without compromising their bio-functionality. Electrostatic bonds are very labile, hydrophobic interactions are easily disrupted and oxidation of sulfhydryl groups is favoured through the application of high pressure processing (HPP) on proteins [15]. This is accompanied by an increase in surface hydrophobicity and formation of disulfide bridges known as protein denaturation leading to aggregation and eventual gelation [16][17][18]. In relatively dilute systems of amaranth protein isolate (5%, w/w), HPP at 200 MPa induced partially unfolding of the protein structure and generated some free sulfhydryl groups [19]. The same pressure intensity increased the water-holding and oil-binding capacity of peanut protein isolate [20]. Extensive conformational changes were recorded upon high pressure application (400 MPa) on amaranth solutions resulting in protein aggregation and the formation of biofilms [17]. Seed and legume protein pressurisation up to 600 MPa showed an excessive formation of high molecular weight and large particle-size protein aggregates, yielding reduced protein solubility in aqueous solutions.
The utilisation of high pressure processing as a method of textural variation requires an understanding of the physicochemical environment, with the ionic strength of solutions featuring prominently. The addition of monovalent ions (Na+), and especially divalent ions (Ca2+ and Mg2+), increases the electrostatic interactions, thereby, inducing a desirable aggregation in the preparation of sweet potato protein [21]. This is due to the binding of positively charged salt ions and negatively charged amino acids of the protein molecule, which is enhanced by HPP at 400 MPa, leading to an improvement in the textural properties of salt-added potato protein in formulations. Care should be taken, though, given that over-salting food could adversely affect the textural behavior following pressurisation, for example, by reducing the hardness, springiness and chewiness of plant protein gels, due to the loss of hydrogen bonding between water molecules and over-aggregated protein [22]. In the case of soy protein isolate, added divalent ions at a neutral pH and pressure of 600 MPa exhibited an increase in solubility ranging from the least effect on ferrous ion-beta conglycinin complex to the highest solubility upon calcium chloride addition [22]. In alkaline solutions, soy protein aggregates in the presence of magnesium or calcium ions could be solubilized after high pressure treatment.
A molecular study of conformational transformation at low levels of soy protein (1%, w/w) and acidic environment (pH 3), following pressurisation at 600 MPa, was carried out by Puppo et al. [23]. The macromolecular characteristics of the pressurized soy protein recorded an increase in surface hydrophobicity, and a decrease in free sulfhydryl content and thermal stability. These outcomes suggested molecular unfolding, with the secondary structure of glycinin turning from a major α-helix in the native state to mainly β-sheets and random coils following application of high pressure. The dissociation of 11S glycinin and 7S β-conglycinin fractions, treated with high pressure, almost disappeared in enthalpy scans, revealing total protein denaturation that supports the aforementioned thermal stability. These structural changes in the glycinin molecule under high pressure affected its gelation and emulsification patterns [24][25].
Soy glycinin was also examined as part of a wider study on the molecular functionality of globular molecules, including whey protein, ovalbumin and BSA, following pressurisation at 600 MPa for 15 min. As shown in Figure 1, this source of plant protein remained largely in the native state (about 20% denaturation) in condensed preparations of 70–80% (w/w) solids that parallels the behaviour of the atmospheric (nonthermally treated) counterparts. The denaturation behaviour of soy glycinin is in between that of whey proteins undergoing almost complete denaturation up to 70% (w/w) solids and BSA that retains native conformation due to the presence of seventeen disulphide bridges in the molecule [26]. Despite the preservation of native conformation, soy glycinin at 80% (w/w) solids is able to form coherent networks exhibiting comparable mechanical strength and glass transition temperature to that of the thermally denatured counterparts, arguing that pressurisation provides both structural functionality and bioactivity in formulation engineering [5].
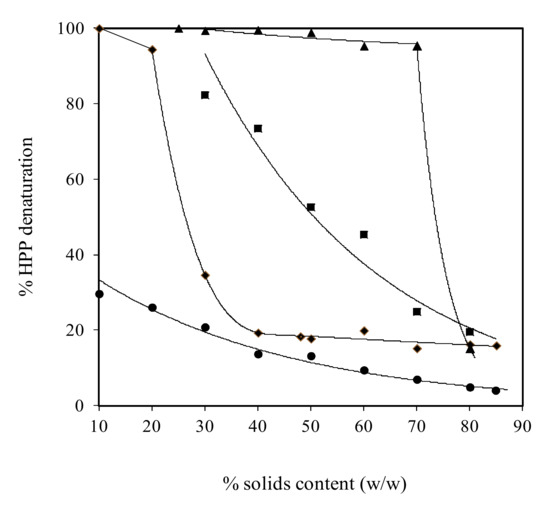
Figure 1. Effect of solids content on the denaturation of pressurised (600 MPa for 15 min) whey protein (▲), soy glycinin (■), ovalbumin (♦) and BSA (●) calculated from denaturation enthalpy (with permission from Savadkhoohi & Kasapis [26]).
1.3. Effect of Ultra-High Temperature (UHT) Processing
This is a hydrothermal process at the elevated temperature of 135–145 °C and a short time of 2–10 s [27] that can improve the solubility [28] and then the gelling capacity [29] of proteins alongside food sterilisation. Jian et al. [30] reported improvement of the solubility from 10 to 95% of soy protein isolate in a two-stage processing, i.e., a low thermal treatment (LT) at 60 °C for 1 h, followed by a UHT treatment at 140 °C for 4 s. The LT treatment enabled the formation of aggregates, held together by non-covalent interactions, while UHT induced primarily the formation of disulphide bridges. This temperature increase, combined with shearing due to steam injection, disrupts various insoluble structures, but encourages the aggregation of 7S and 11S molecular fractions [28]. Figure 2 illustrates dramatic changes in the elastic properties of soy protein in a small-deformation oscillatory test used to confirm the process of gelation, following the aforementioned double-thermal treatment (LT + UHT). Heating and equilibration at 90 °C for 30 min followed by cooling to 20 °C resulted in a significant increase in elastic modulus (G′) that exceeded fivefold the mechanical strength of the soy gel subjected only to LT treatment. It should be noted, however, that in pea protein isolate (PPI), a relatively low solubility (up to 55% of an 8% w/w preparation at pH 7.5) has been reported following a similar UHT treatment caused by the presence of insoluble non-covalent aggregates [31].
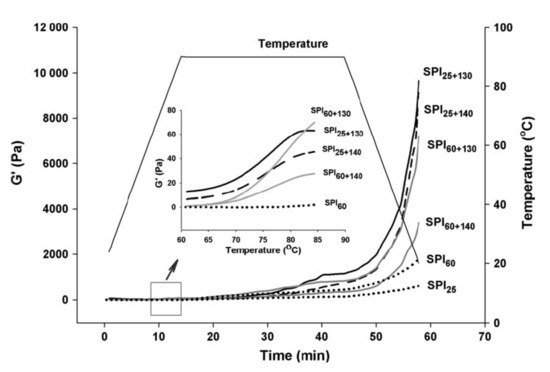
Figure 2. Typical curves of elastic modulus (G′) of soy protein isolate subjected to isothermal and two-stage heating processes plotted against temperature (heating, holding and cooling). The subscript values of 25, 60, 130 and 140 denote heating temperatures (°C) with a holding time of 1 h at 25 and 60 °C (LT), or 4 s at 130 and 140 °C (UHT). The inset magnifies the onset of gelation with rising temperature (with permission from Jian et al. [30]).
Plant proteins from cereals, legumes and nuts have been subjected to high pressure homogenization prior to UHT treatment in an effort to reduce their particle size known as ultra high pressure homogenization (UHPH) at 300 MPa [32][33][34]. UHT treatment (140 °C, 2 s) following low-pressure homogenization at 50 MPa (UHT + LPH), autoclave sterilization (AC) at 121 °C for 15 min, and ultra high-pressure homogenization with low thermal treatment at 300 MPa and 50 °C (UHPH + LT) unveiled the physical profile of soy milk. This focused on particle size, degree of protein denaturation, aggregation rate and gel-network density index, which could improve the sensory profile of preparations [35]. Similar outcomes were reported for pea protein isolate, including a UHPH + UHT treatment (500 bar, 140 °C, 2 s) by Qamar, Bhandari and Prakash [36].
In addition, UHT affects the Kunitz trypsin inhibitor in soybeans, a potent protease inhibitor to trypsin and chymotrypsin binding in the human gut. High temperature application (135–150 °C) for 10–50 s inactivated the trypsin inhibitor due to protein aggregation, which is further increased in the presence of sodium chloride [37][38][39]. It was reported that 90% of trypsin inhibitor could be destroyed by a single step of UHT at 143 °C, 60 s or a two-step process of UHT at 143°C, 4 s following low-temperature long-time heating (95 °C, 60 min), with the treated soymilk possessing highly acceptable colour, flavor and vitamin content [40]. The mechanism of UHT-induced inactivation of Kunitz trypsin inhibitor is attributed to alterations in the conformation of the protein by increasing the content of disulfide bonds and non-covalent interactions. In comparison, Bowman-Birk inhibitor, another trypsin inhibitor in soybean, has a higher heat stability to UHT, based on a molecular structure of seven disulfide bonds in the highly hydrophilic protein. The tight conformation diminishes the thermal aggregation of Bowman-Birk inhibitor, leading to loss of bioactivity, but sodium chloride addition can disturb various molecular associations causing inactivation of the inhibitor [37].
With respect to the development of an unpleasant sensory profile in plant protein systems, lipoxygenase is an enzyme that interacts with off-flavour precursors, mainly unsaturated fatty acids, resulting in lipid oxidation. Numerous volatile compounds, e.g., alcohols, aldehydes, ketones and esters are generated, and are capable of deteriorating the organoleptic property of soy protein at low concentrations (1–5 ppb) [41]. Soybean protein is naturally bound to lipoxygenase via non-covalent interactions, and how-temperature heating activates the conformation of the enzyme to act on linoleic acid and initiate off flavour development [42][43]. UHT processing can diminish off-flavour oxidation processes due to enzyme inactivation and soy protein denaturation. That was reported in plant-based dairy substitutes utilised in the manufacture of liquid breakfast that was treated at 145 °C for 8 s and sampled over a short shelf-life of 90 days at 20 and 30 °C [44].
1.4. Effect of Ultrasound Treatment
Ultrasonication is a preservation tool that aims to minimise processing but at the same time increase quality and safety of food products [45]. Low-energy (low power, low intensity) ultrasound has frequencies higher than 100 kHz at intensities below 1 W/cm2 while high-energy (high power, high-intensity) ultrasound uses intensities higher than 1 W/cm2 at frequencies between 20 and 500 kHz [46]. In plant protein studies, low frequency ultrasound of 20–100 kHz and power in the range 100–1000 W/cm2 has altered the structural property of plant proteins due to strong shear and mechanical forces, occurring in the cavitation phenomenon [47][48]. This phenomenon induces changes in protein conformation by exposing sulfhydryl groups and increasing the hydrophobic patches of the protein surface hence influencing plant protein functionality.
The impact of ultrasound treatment on plant protein isolates depends on two parameters, i.e., ultrasound intensity [48][49] and ultrasound exposure time [48][50][51][52]. High intensity of the ultrasound wave could induce aggregation of numerous plant protein extracts (pea, canola, album seed) suspended in an aqueous solution. Longer sonication period encouraged protein-water interactions resulting in a broad size distribution of fractured microparticles in the protein suspension [49][50][52].
Studies on a soy protein isolate (SPI) dispersion, treated with low-frequency (20 kHz) ultrasonication, at different powers (200, 400 or 600 W) and duration of treatment (15 or 30 min), revealed that longer treatment time and intensity of power reduced the value of elastic modulus (G′) significantly but extended the linear viscoelastic region (LVR) of the rediluted 12.5% SPI dispersion in a strain test (Figure 3a) [53]. Similarly, the value of elastic (G′) and loss (G″) modulus of the ultrasound treated SPI dispersion was lower compared to the non-treated one, accompanied by an order of magnitude increase in moduli from 0.1 to 1 Pa with increasing oscillatory frequency from 0.1 to 100 rad/s. Findings indicated the flow behaviour of SPI with ultrasonic treatment compared to the non-treated sample, with liquid–like properties being more pronounced for the samples treated with low-power (200 W) at a short period of time (15 min) (Figure 3b,c).
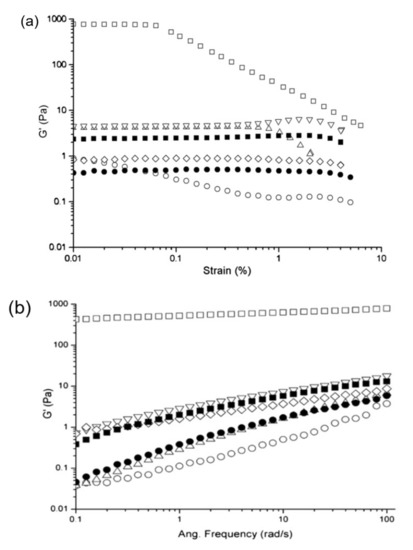
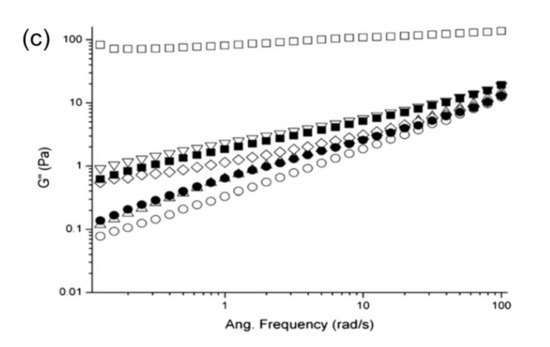
Figure 3. (a) Strain sweep, (b) elastic modulus (G′), and (c) loss modulus (G″) of a 12.5% soy protein dispersion having different ultrasonic treatment of no ultrasound (□); 200W for 15 min (◯); 200W for 30 min (△); 400 W for 15 min (▽); 400 W for 30 min (◇); 600 W for 15 min (■); and 600 W for 30 min (●) (with permission from Hu et al. [53]).
On the other hand, ultrasound treatment did not affect the primary structure of numerous legume proteins shown in segmented molecular weight fingerprints analysed using protein gel electrophoresis [49][50][53][54][55]. It can reduce the molecular weight, for example in pea protein (32–55 kDa in Mir et al. [52]) and millet proteins (40–50 kDa in Nazari et al. [47]). However, ultrasonication affected the secondary structure of millet protein isolate, investigated by Fourier-transform infrared spectra (FTIR), zeta-potential values and thermal analysis. The main change in Amide I region (1700–1600 cm−1) was reflected in the intensity of C = O stretching vibrations of the peptide bonds in millet protein dispersions of 10% solids. Shift of Amide II bands (1530–1550 cm−1) following ultrasound treatment was attributed to the partial transformation of random coil to predominantly β-sheet.
The positional change in Amide II region of millet protein decreased the intermolecular hydrogen bonds, while increased the quantity of negative charges, as recorded by zeta potential values [47]. Disruption of the secondary structure affected thermal stability seen in a decrease in enthalpy of denaturation by DSC heating to 130 °C at 10 °C /min or loss in the weight of protein mass during thermogravimetric analysis by heating to 800 °C at 10 °C/min. In terms of the pea protein dispersion (also 10% solids), electrostatic repulsion showed a converse result to that of millet protein, where the net negative charge was lower in ultrasound treatment due to its distinct secondary structure [49]. In general, the work confirmed the cavitational and turbulent effects of ultrasonication on breaking down high molecular weight into small size particles and the destruction of intermolecular hydrogen bonds in plant protein structure [47][52].
High-solid systems with improved properties were produced using high intensity ultrasound treatment on relatively dilute protein solutions prior to film formation. Ultrasound application allowed the formation of homogeneous protein dispersions via increasing hydrogen bond formation with water molecules. A casting technique was then utilised to prepare thin films at high levels of solids (about 90% w/w). In cases of both, gluten and soy-protein based films, the large deformation properties of tensile strength and film elongation were considerably enhanced [56][57]. In addition, composites with a synthetic polymer, polyacrylamide, were prepared in an effort to further manipulate the mechanical properties of soybean protein isolate by varying three experimental conditions: Sonication power, sonication time and slurry temperature [58]. Sonication treatment power varied from 200 to 600 W and generated variable cavitation and microstreaming to rearrange and unfold the soy protein molecule. The film structure, produced by the sonication, showed a higher adhesion force and tensile strength but lower film elongation due to increasing stress effects on the soy protein/polyacrylamide interface in the phase separated mixture. It was also observed that application of sonication power at more than 500 W reduced intermolecular cohesion amongst the polymeric constituents in the matrix by disrupting hydrogen bonding, hydrophobic and electrostatic interactions that led to increased polymer/water interactions.
Low-frequency ultrasound has been a potential non-thermal technique for the improvement of the functional properties of plant-sourced proteins achieving quality at parity to that of animal sources [55]. Functionality mainly focuses on colour, solubility, foaming property, emulsification and gelation. Application of an ultrasound wave increased the whiteness of protein isolate from Chenopodium album seed due to the destruction of protein-pigment complexation [52]. The solubility of treated black bean protein isolates was also improved by converting large surface area-to-small particle sizes in protein dispersions and exposing polar surfaces [54]. Sonication increased the water holding capacity of protein in Ganxet beans [59], but partially denatured canola protein by exposing hydrophobic regions to support gelation [50].
Several studies showed high foaming capacity and foam stability of flexible plant-protein secondary structures in aqueous solutions due to an intense homogenization via ultrasound treatment, which increased the surface hydrophobicity and protein diffusion in air-water interfaces. Plant protein foam exhibited the characteristics of a thick and cohesive layer covering air bubbles following stabilization with high-intensity ultrasound waves [49][50][52]. Emulsification of soy protein isolates was also enhanced with various ultrasonication protocols. Emulsion droplets were small in comparison to untreated counterparts, allowing the adsorption of protein at oil-water interfaces with a lower interfacial surface tension. In addition, ultrasound-treated soy protein emulsions were subjected to shelf-life studies to show good stability under storage for 28 days [60].
References
- Savadkoohi, S.; Bannikova, A.; Kasapis, S. Glass Transition of Globular Proteins from Thermal and High Pressure Perspectives. In Glass Transition and Phase Transitions in Food and Biological Materials; Jasim, A., Ed.; Wiley: New York, NY, USA, 2017; pp. 49–117.
- Ma, L.; Cui, X.; Cai, W.; Shao, X; Understanding the function of water during the gelation of globular proteins by temperature-dependent near infrared spectroscopy. Physical Chemistry Chemical Physics 2018, 20, 20132–20140, 10.1039/c8cp01431k.
- Schmidt, R.H. Gelation and Coagulation. In Protein Functionality in Foods; American Chemical Society: Washington, DC, USA, 1981; Volume 147, pp. 131–147.
- Taco Nicolai; Christophe Chassenieux; Heat-induced gelation of plant globulins. Current Opinion in Food Science 2019, 27, 18-22, 10.1016/j.cofs.2019.04.005.
- Sobhan Savadkoohi; Anna Bannikova; Nitin Mantri; Stefan Kasapis; Structural modification in condensed soy glycinin systems following application of high pressure. Food Hydrocolloids 2016, 53, 115-124, 10.1016/j.foodhyd.2014.07.028.
- Khetan Shevkani; Narpinder Singh; AmritPal Kaur; Jai Chand Rana; Structural and functional characterization of kidney bean and field pea protein isolates: A comparative study. Food Hydrocolloids 2015, 43, 679-689, 10.1016/j.foodhyd.2014.07.024.
- Sok Li Tay; Stefan Kasapis; Conrad Perera; Philip J. Barlow; Functional and Structural Properties of 2S Soy Protein in Relation to Other Molecular Protein Fractions. Journal of Agricultural and Food Chemistry 2006, 54, 6046-6053, 10.1021/jf060387a.
- J.A.M. Berghout; R.M. Boom; A.J. Van Der Goot; Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocolloids 2015, 43, 465-472, 10.1016/j.foodhyd.2014.07.003.
- Allaoua Achouri; Vincent Nail; Joyce Irene Boye; Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Research International 2012, 46, 360-369, 10.1016/j.foodres.2012.01.001.
- Bruce German; Srinivasan Damodaran; John E. Kinsella; Thermal dissociation and association behavior of soy proteins. Journal of Agricultural and Food Chemistry 1982, 30, 807-811, 10.1021/jf00113a002.
- Outi Mäkinen; Emanuele Zannini; Peter Koehler; Elke K. Arendt; Heat-denaturation and aggregation of quinoa ( Chenopodium quinoa ) globulins as affected by the pH value. Food Chemistry 2016, 196, 17-24, 10.1016/j.foodchem.2015.08.069.
- Douglas Wong; Thava Vasanthan; Lech Ozimek; Synergistic enhancement in the co-gelation of salt-soluble pea proteins and whey proteins. Food Chemistry 2013, 141, 3913-3919, 10.1016/j.foodchem.2013.05.082.
- Carsten Ersch; Irene Ter Laak; Erik Van Der Linden; Paul Venema; Anneke Martin; Modulating fracture properties of mixed protein systems. Food Hydrocolloids 2015, 44, 59-65, 10.1016/j.foodhyd.2014.09.009.
- Juliana V.C. Silva; Rémy Cochereau; Christophe Schmitt; Christophe Chassenieux; Taco Nicolai; Heat-induced gelation of mixtures of micellar caseins and plant proteins in aqueous solution. Food Research International 2019, 116, 1135-1143, 10.1016/j.foodres.2018.09.058.
- Rui Queirós; Jorge A. Saraiva; José Lopes Da Silva; Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Critical Reviews in Food Science and Nutrition 2018, 58, 1538-1556, 10.1080/10408398.2016.1271770.
- Jasim Ahmed; Noor Al-Ruwaih; Mehraj Fatema Mulla; Muhammad H. Rahman; Effect of high pressure treatment on functional, rheological and structural properties of kidney bean protein isolate. LWT 2018, 91, 191-197, 10.1016/j.lwt.2018.01.054.
- María Cecilia Condés; Maria Cristina Añón; Adriana Noemí Mauri; Amaranth protein films prepared with high-pressure treated proteins. Journal of Food Engineering 2015, 166, 38-44, 10.1016/j.jfoodeng.2015.05.005.
- F. Peyrano; Francisco Speroni; Maria V. Avanza; Physicochemical and functional properties of cowpea protein isolates treated with temperature or high hydrostatic pressure. Innovative Food Science & Emerging Technologies 2016, 33, 38-46, 10.1016/j.ifset.2015.10.014.
- María Cecilia Condés; Francisco Speroni; Adriana Noemí Mauri; Maria Cristina Añón; Physicochemical and structural properties of amaranth protein isolates treated with high pressure. Innovative Food Science & Emerging Technologies 2012, 14, 11-17, 10.1016/j.ifset.2011.12.006.
- Xuan-Hui He; Hongzhi Liu; Li Liu; Guan-Li Zhao; Qiang Wang; Qiong-Ling Chen; Effects of high pressure on the physicochemical and functional properties of peanut protein isolates. Food Hydrocolloids 2014, 36, 123-129, 10.1016/j.foodhyd.2013.08.031.
- Zhong-Kai Zhao; Taihua Mu; Miao Zhang; Aurore Richel; Effect of salts combined with high hydrostatic pressure on structure and gelation properties of sweet potato protein. LWT 2018, 93, 36-44, 10.1016/j.lwt.2018.03.007.
- Carlos Alberto Manassero; Sergio R. Vaudagna; Maria Cristina Añón; Francisco Speroni; High hydrostatic pressure improves protein solubility and dispersion stability of mineral-added soybean protein isolate. Food Hydrocolloids 2015, 43, 629-635, 10.1016/j.foodhyd.2014.07.020.
- Cecilia Puppo; Nicolas Chapleau; Francisco Speroni; Marie De Lamballerie-Anton; F. Michel; Cristina Añón; Marc Anton; Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates. Journal of Agricultural and Food Chemistry 2004, 52, 1564-1571, 10.1021/jf034813t.
- Katsuyoshi Nishinari; Ya-Peng Fang; S. Guo; G.O. Phillips; Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocolloids 2014, 39, 301-318, 10.1016/j.foodhyd.2014.01.013.
- Xian-Sheng Wang; Chuan-He Tang; Bian-Sheng Li; Xiao-Quan Yang; Ling Li; Ching-Yung Ma; Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloids 2008, 22, 560-567, 10.1016/j.foodhyd.2007.01.027.
- Sobhan Savadkoohi; Stefan Kasapis; High pressure effects on the structural functionality of condensed globular-protein matrices. International Journal of Biological Macromolecules 2016, 88, 433-442, 10.1016/j.ijbiomac.2016.04.012.
- Rupesh S. Chavan; Shraddha Rupesh Chavan; Chandrashekar D. Khedkar; Atanu Jana; UHT Milk Processing and Effect of Plasmin Activity on Shelf Life: A Review. Comprehensive Reviews in Food Science and Food Safety 2011, 10, 251-268, 10.1111/j.1541-4337.2011.00157.x.
- Heng-Guang Zheng; Xiao-Quan Yang; Chuan-He Tang; Lin Li; Ijaz Ahmad; Preparation of soluble soybean protein aggregates (SSPA) from insoluble soybean protein concentrates (SPC) and its functional properties. Food Research International 2008, 41, 154-164, 10.1016/j.foodres.2007.10.013.
- Takao Nagano; Youichi Fukuda; Takeshi Akasaka; Dynamic Viscoelastic Study on the Gelation Properties of β-Conglycinin-Rich and Glycinin-Rich Soybean Protein Isolates. Journal of Agricultural and Food Chemistry 1996, 44, 3484-3488, 10.1021/jf9507497.
- Huajun Jian; Youling L. Xiong; Fengxian Guo; Xiaolin Huang; Benu Adhikari; Jie Chen; Gelation enhancement of soy protein isolate by sequential low- and ultrahigh-temperature two-stage preheating treatments. International Journal of Food Science & Technology 2014, 49, 2529-2537, 10.1111/ijfs.12694.
- Dimuthu Bogahawaththa; Nguyen Hoang Bao Chau; Jigar Trivedi; Muditha Dissanayake; Todor Vasiljevic; Impact of selected process parameters on solubility and heat stability of pea protein isolate. LWT 2019, 102, 246-253, 10.1016/j.lwt.2018.12.034.
- Outi Mäkinen; Therese Uniacke-Lowe; James A. O’Mahony; Elke K. Arendt; Physicochemical and acid gelation properties of commercial UHT-treated plant-based milk substitutes and lactose free bovine milk. Food Chemistry 2015, 168, 630-638, 10.1016/j.foodchem.2014.07.036.
- David Julian McClements; Emily Newman; Isobelle Farrell McClements; Plant‐based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Comprehensive Reviews in Food Science and Food Safety 2019, 18, 2047-2067, 10.1111/1541-4337.12505.
- Swati Sethi; S. K. Tyagi; Rahul K. Anurag; Plant-based milk alternatives an emerging segment of functional beverages: a review. Journal of Food Science and Technology 2016, 53, 3408-3423, 10.1007/s13197-016-2328-3.
- Nelly Del Socorro Cruz-Cansino; Marta Capellas; D.P. Jaramillo; Antonio-José Trujillo; B. Guamis; Victoria Ferragut; Soymilk treated by ultra high-pressure homogenization: Acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocolloids 2009, 23, 490-496, 10.1016/j.foodhyd.2008.03.010.
- Sadia Qamar; Bhesh Bhandari; Sangeeta Prakash; Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Research International 2019, 116, 1374-1385, 10.1016/j.foodres.2018.10.028.
- Yeming Chen; Zhicun Xu; Caimeng Zhang; Xiangzhen Kong; Yufei Hua; Heat-induced inactivation mechanisms of Kunitz trypsin inhibitor and Bowman-Birk inhibitor in soymilk processing. Food Chemistry 2014, 154, 108-116, 10.1016/j.foodchem.2013.12.092.
- Kwok, K.-C.; Niranjan, K; Review: Effect of thermal processing on soymilk. Int. J. Food Sci. Technol. 1995, 30, 263-295, 10.1111/j.1365-2621.1995.tb01377.x.
- Shaohong Yuan; Sam K. C. Chang; Zhisheng Liu; Baojun Xu; Elimination of Trypsin Inhibitor Activity and Beany Flavor in Soy Milk by Consecutive Blanching and Ultrahigh-Temperature (UHT) Processing. Journal of Agricultural and Food Chemistry 2008, 56, 7957-7963, 10.1021/jf801039h.
- Kin-Chor Kwok; Hanhua Liang; Keshavan Niranjan; Optimizing Conditions for Thermal Processes of Soy Milk. Journal of Agricultural and Food Chemistry 2002, 50, 4834-4838, 10.1021/jf020182b.
- Srinivasan Damodaran; Akshay Arora; Off-Flavor Precursors in Soy Protein Isolate and Novel Strategies for their Removal. Annual Review of Food Science and Technology 2013, 4, 327-346, 10.1146/annurev-food-030212-182650.
- Ostergaard Alsoe, K.; Adler-Nissen, J. Thermal denaturation of lipoxygenase from soya beans in the presence of its natural substrate; Dénaturation thermique de la lipoxygénase de soja en présence de son substrat naturel. Leb. - Wiss. + Technol. 1988, 21, 8–12.
- Youru Huang; Yufei Hua; Aiyong Qiu; Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Research International 2006, 39, 240-249, 10.1016/j.foodres.2005.07.012.
- Nashi K. Alqahtani; John Ashton; Lita Katopo; Elisabeth Gorczyca; Stefan Kasapis; Shelf-life studies of flavour characteristics in model UHT liquid systems enriched with wholegrain oat.. Heliyon 2018, 4, e00566, 10.1016/j.heliyon.2018.e00566.
- Ishrat Majid; Gulzar Nayik.; Vikas Nanda; Ultrasonication and food technology: A review. Cogent Food & Agriculture 2015, 1, 1071022, 10.1080/23311932.2015.1071022.
- Timothy James Mason; F. Chemat; Mircea Vinatoru; The Extraction of Natural Products using Ultrasound or Microwaves. Current Organic Chemistry 2011, 15, 237-247, 10.2174/138527211793979871.
- Bahman Nazari; Mohammad Amin Mohammadifar; Saeedeh Shojaee-Aliabadi; Ehsan Feizollahi; Leila Mirmoghtadaie; Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrasonics Sonochemistry 2018, 41, 382-388, 10.1016/j.ultsonch.2017.10.002.
- Moxi Zhou; Jian Liu; Yuanyuan Zhou; Xingjian Huang; Fengxia Liu; Siyi Pan; Hao Hu; Effect of high intensity ultrasound on physicochemical and functional properties of soybean glycinin at different ionic strengths. Innovative Food Science & Emerging Technologies 2016, 34, 205-213, 10.1016/j.ifset.2016.02.007.
- Ting Xiong; Wenfei Xiong; Mengting Ge; Junhao Xia; Bin Li; Yijie Chen; Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Research International 2018, 109, 260-267, 10.1016/j.foodres.2018.04.044.
- Nitzia Thalía Flores-Jiménez; José Armando Ulloa; Judith Esmeralda Urías Silvas; José Carmen Ramírez Ramírez; Petra Rosas Ulloa; Pedro Ulises Bautista Rosales; Yessica Silva Carrillo; Ranferi Gutiérrez Leyva; Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate.. Food Research International 2019, 121, 947-956, 10.1016/j.foodres.2019.01.025.
- Hao Hu; Imelda W.Y. Cheung; Siyi Pan; Eunice C.Y. Li-Chan; Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocolloids 2015, 45, 102-110, 10.1016/j.foodhyd.2014.11.004.
- Nisar A. Mir; C S Riar; Sukhcharn Singh; Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocolloids 2019, 96, 433-441, 10.1016/j.foodhyd.2019.05.052.
- Hao Hu; Jiahui Wu; Eunice C.Y. Li-Chan; Le Zhu; Fang Zhang; Xiaoyun Xu; Gang Fan; Lufeng Wang; Xingjian Huang; Siyi Pan; Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocolloids 2013, 30, 647-655, 10.1016/j.foodhyd.2012.08.001.
- Lianzhou Jiang; Jing Wang; Yang Li; Zhongjiang Wang; Jing Liang; Rui Wang; Yong Chen; Wenjun Ma; Baokun Qi; Min Zhang; Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Research International 2014, 62, 595-601, 10.1016/j.foodres.2014.04.022.
- Jonathan O'sullivan; Brian Murray; Cal Flynn; Ian Norton; The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids 2016, 53, 141-154, 10.1016/j.foodhyd.2015.02.009.
- Eva Marcuzzo; Donatella Peressini; Frédéric Debeaufort; Alessandro Sensidoni; Effect of ultrasound treatment on properties of gluten-based film. Innovative Food Science & Emerging Technologies 2010, 11, 451-457, 10.1016/j.ifset.2010.03.002.
- Yang Sun; Chun Yu Sun; Guang Chen; Effect of Ultrasound Treatment on Properties of Soy Proteins Film. Applied Mechanics and Materials 2011, 117, 513-516, 10.4028/www.scientific.net/amm.117-119.513.
- Xinwei Cao; Bo Zhu; Yichuan Gao; Jianli Liu; Weidong Gao; Xiaoling Gai; Wei Bao; Process optimization of ultrasound-assisted treatment for soya bean protein isolate/polyacrylamide composite film. Royal Society Open Science 2018, 5, 180213, 10.1098/rsos.180213.
- Tomás Lafarga; Carlos Álvarez; Gloria Bobo; Ingrid Aguiló-Aguayo; Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT 2018, 98, 106-112, 10.1016/j.lwt.2018.08.033.
- Jonathan O'sullivan; Michael Park; Jack Beevers; The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. Journal of Cereal Science 2016, 69, 77-84, 10.1016/j.jcs.2016.02.013.




