1000/1000
Hot
Most Recent

Bioactive lipids are a newly defined class of lipids that are actively involved in the regulation of a variety of cellular events. As the name indicates, these molecular factors are subjected to action at the arrival of a specific stimulus and undergo subsequent transitions to cope up with the insult. Among these bioactive lipids, sphingolipids have emerged as distinctive mediators of various cellular processes, ranging from cell growth and proliferation to cellular apoptosis, executing immune responses to regulating inflammation. Recent studies have made it clear that sphingolipids, their metabolic intermediates (ceramide, sphingosine-1-phosphate, and N-acetyl sphingosine), and enzyme systems (cyclooxygenases, sphingosine kinases, and sphingomyelinase) harbor diverse yet interconnected signaling pathways in the central nervous system (CNS), orchestrate CNS physiological processes, and participate in a plethora of neuroinflammatory and neurodegenerative disorders.
In addition to being the major components of the cell membrane, bioactive lipids function as signaling molecules and possess diverse functional capabilities. Bioactive lipids mediate a plethora of physiological roles, have a relatively short half-life, and act through G-protein-coupled receptors. As mentioned earlier, the subgroups of bioactive lipids contribute toward heterogeneous functions in the body, are recruited at the time of need, and undergo subsequent transitions to deal with the challenge. These lipids are synthesized from omega-3 polyunsaturated fatty acids (PUFAs) and arachidonic acid (AA) and are acted upon by various enzymes such as cyclooxygenases (COXs), lipoxygenases (LOXs), ceramidases, sphingosine kinases (Sphks), and many others. These enzyme systems, and their subsequent expression and mechanistic pathways followed, are crucial in monitoring these lipid classes and their functional potential [1].
Eicosanoids play a role in modulating the inflammatory and immune responses in that they secrete inflammatory cytokines and chemo-attractants, recruit leukocytes, and regulate blood flow to the site of insult. This lipid class is further composed of prostanoids (prostaglandins (PGs), prostacyclins, and thromboxanes), leukotrienes, and lipoxins [2]. Eicosanoids are ubiquitously synthesized by the body, i.e., each cell has the machinery to generate one or two major eicosanoids, which in turn function in an autocrine and paracrine manner to maintain local cellular and tissue homeostasis. They act as dynamic regulators of blood pressure homeostasis as they possess the capacity of both vasodepressors (molecules that cause vasomotor depression leading to a reduction in blood pressure) and vasopressors (group of molecules that contract blood vessels and elevate blood pressure) [3]. They also regulate a wide variety of neurophysiological functions such as body temperature regulation, hormonal release, sleep–wake cycle, pain, and inflammatory responses [4]. Some eicosanoids are secreted by the uterus, which then act on the corpus luteum (a temporary endocrine structure in the ovaries responsible for progesterone secretion), causing uterine contractions, otherwise known as labor [5]. These findings demonstrate the significance of eicosanoids in maintaining body homeostasis and the mechanism through which these lipids regulate the physiology of the body from the CNS to the reproductive organs.
Phospholipids are the major components of cell membranes based on their structural configuration as they possess both hydrophilic and hydrophobic ends that automatically arrange themselves into membranes. Phospholipid subgroups include phosphatidylcholine (PC), phosphatidylserine (Ptd-L-Ser or PS), phosphatidylinositol (PI), and many others. These lipid classes provide the cells with barrier properties and structural integrity and also monitor membrane trafficking. They permit cells to be selectively permeable to extracellular substances, thus maintaining the cellular homeostatic environment [6]. In addition to contributing toward structural properties, phospholipids ensure cell survival and growth, play a role in endocytosis, phagocytosis [7], and immune surveillance [8], as well as acting as lipid transporters. The CNS is highly enriched in phospholipids, playing a critical role in synaptic transmission, improving cognition, increasing stress resistance, and exerting antioxidant activity [9][10]. These observations significantly indicate the critical role of phospholipids not only in membrane biology but also in other systems, such as the brain.
Sphingolipids, besides providing the physiochemical properties to cell membranes, also modulate crucial signaling processes. Sphingolipid metabolic intermediates, including ceramide, sphingosine, and sphingosine-1-phosphate (S1P), are the most explored bioactive sphingolipids. The intracellular and intercellular expression of sphingolipids is modulated by multifaceted complex enzyme systems, among which ceramidase, Sphks (Sphk1 and Sphk2), and S1P lyase are the major regulators [11]. These molecules exert a variety of functions that comprise the regulation of cellular growth, apoptosis, immune cell responses, inflammation, and cell survival. Ceramide has the potential to regulate cell growth, differentiation, cellular senescence, and apoptosis. However, sphingosine has been demonstrated to act as a repressor of cell growth and participate in cell arrest and apoptosis. S1P has antagonizing effects similar to those of ceramide, such as promoting cell survival, proliferation, and resistance to cell apoptosis as well as modulating vasculogenesis, angiogenesis, and inflammation [12][13]. These findings state the substantial significance of sphingolipids in a wide range of physiological functions in the body and emphasize the diversified nature of their metabolites.
SPMs are a relatively newly discovered class of bioactive lipids possessing immunoresolvent and anti-inflammatory properties and comprise lipoxins, resolvins, protectins, and maresins. Like other lipid classes, SPMs are endogenously synthesized from PUFAs in response to inflammation or injury. They primarily function to resolve the inflammatory response by restricting the infiltrating immune cells, inhibiting pro-inflammatory cytokines secretion, promoting efferocytosis, and removing cellular debris [14]. Each member of this class not only has a distinct chemical structure but also exerts peculiar functions. For instance, lipoxins obstruct neutrophil infiltration at the site of inflammation, promote macrophage-regulated efferocytosis, and recruit anti-inflammatory monocytes [15]. Resolvins impede the synthesis of pro-inflammatory cytokines, limit leukocyte trafficking, and clear cell debris [16]. However, protectins have neuroprotective properties as well as anti-inflammatory and pro-apoptotic features [17]. Maresins are specifically released by macrophages and are involved in the macrophage-induced phagocytic activity and resolution of inflammation [18]. These studies support the notion that SPMs, along with other classes of bioactive lipids, are critically important in modulating body homeostasis and maintaining the normal physiology.
One of the potential mechanisms that Aβ deposition disrupts during Alzheimer’s disease (AD) pathogenesis is the deregulation of neuronal Sphk1–S1P signaling in the CNS. Cellular studies have reported that a shift in sphingolipid rheostat, i.e., the transition from a cell survival mediator (S1P) to a pro-apoptotic ceramide, occurs during AD pathogenesis [19]. Elevated levels of ceramides have been reported, while a reduction in neuronal S1P levels has been observed along with alterations in Sphk1 enzyme concentrations. The Sphk1-modulated sphingolipid metabolism disruption is severely highlighted in the etiology of AD mice models, and abnormally altered levels of ceramides and S1P are observed in patients with AD [20].
Postmortem studies on patients with AD have reported a reduction in neuronal Sphk1 activity, which consequently monitors S1P levels in the brain. Sphk1 and S1P are affected by Aβ deposition in the brain, where Sphk1–S1P signaling inversely correlates with increasing Aβ concentrations, i.e., the more the Aβ is deposited, the lesser is the Sphk1–S1P activity. Conversely, S1P lyase (the enzyme that regulates the final step of S1P catalytic conversion into end products) is elevated in AD pathology and exhibits a positive correlation with Aβ deposition, i.e., the greater the Aβ pathology, the higher the S1P lyase activity and the lower the S1P levels in the brain [21]. This overall shift in sphingolipid metabolism results in lower levels of S1P in the brain, which is a pro-survival and neuroprotective metabolite. This transition leads to sustained neuroinflammation and exaggerated neuronal loss. In addition, an alteration in Sphk1–S1P activity has been found to be dominant in some CNS regions compared with that in other regions, i.e., decreased S1P expression, reduced Sphk1 activity, and elevated S1P lyase levels were detected in the CA1 region of the hippocampus and gray and white matter of the temporal gyrus in patients with AD; both these regions regulate memory formation and spatial learning in the brain [20]. In vitro studies of reciprocating AD pathology through treatment of neuronal cells with Aβ peptide have also demonstrated similar patterns to those of Sphk1–S1P signaling. Aβ-treated neuronal cells were found to exhibit elevated ceramide concentrations, reduced Sphk1 activity and S1P levels, and enhanced cellular apoptosis. However, overexpression of Sphk1 conferred neuronal cell cytoprotection, restored the sphingolipid balance toward neuroprotective S1P, and reduced cell death [22].
Altogether, these findings provide a potential direction for evaluating AD pathology in view of sphingolipid metabolism, with a particular focus on the protective role of neuronal Sphk1–S1P signaling. Although this Sphk1–S1P signaling has been investigated in AD pathogenesis, recent research has indicated potential new roles of Sphk1 in both CNS physiology and pathology. The latest findings have suggested a new functional characteristic of Sphk1, i.e., Sphk1 acts as an acetyltransferase for COX2 in the brain, and have mentioned the crucial contribution of the Sphk1 and COX2 bridging connection in the CNS.
In the steady-state CNS, a perfectly harmonious coordination of Sphk1 and COX2 is present, which plays a very crucial role in the maintenance of the homeostatic environment, and disruption of this harmony results in neuroinflammation and eventually neurodegeneration. During physiological conditions, Sphk1 is expressed by various brain regions and neuronal cells, and it regulates diverse cellular processes, e.g., neurogenesis, axonal growth, and the release of neurotransmitters [23][24]. Sphk1 in the brain has different signaling pathways, such as ATP-dependent sphingosine metabolism to S1P, Sphk1–S1P signaling, and modulation of COX2 expression by functioning as an acetyltransferase. It has been recently reported that Sphk1 has the ability to catalytically modify COX2 and acetylate it at the serine 565 site. As mentioned earlier, COX2 has both inflammatory and anti-inflammatory activity and, depending on the stimulating cue and the enzymes modulating its activity, can result in either of two characteristics. During the inflammatory response, COX2 generates a large number of prostanoids, which sustains the ongoing insult [25]. Non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin are the first drug of choice used to treat inflammatory diseases. The major pathway followed by aspirin is acetylation of COX2 and shifting its activity toward the anti-inflammatory domain, which consequently synthesizes the immunoresolvent SPMs [26]. This mechanism is also observed in animal studies, where Sphk1 functions as an endogenous regulator of COX2 acetylation and SPM secretion, rather than exogenously administered drugs.
This triad of Sphk1–COX2–SPMs is severely altered in AD pathogenesis in both animal models and human studies. A recent study showed that neuronal Sphk1 expression was reduced in AD mice compared with that in the control group. The brains of AD mice displayed reduced mRNA expression of neuronal Sphk1, but no considerable difference was observed between the glial cell population, which highlights the cell-dependent activity of Sphk1. Due to reduced Sphk1 expression and activity, the COX2 anti-inflammatory function and the synthesis of SPMs were significantly disturbed. The study also reported that during AD pathology, Sphk1 expression was decreased, shifting the COX2 activity toward prostanoid synthesis and impeding the production of SPMs [27]; in short, this explains the poor resolution of inflammation due to the abrogated communication between Sphk1, COX2, and SPMs. The dysfunction of this trilogy causes a dysregulated crosstalk between neuronal and glial cells, affects the microglial phagocytosis of Aβ, increases the production of pro-inflammatory cytokines such as TNFα, IL-1β, and IL-6, and fails to inhibit the infiltration of peripheral immune cells into the CNS. These abnormalities, along with reduced SPM release, sustain the neuroinflammatory phenotype of the AD brain, increase the Aβ load, and eventually lead to neurodegeneration.
However, elevated levels of neuronal Sphk1 improved the AD pathology by restoring the COX2 anti-inflammatory activity, synthesizing neuronal SPMs, and reestablishing neuronal and glial cell communication. Increased Sphk1 levels also reduced the COX2-triggered prostanoid secretion by modifying its catalytic domain at serine 565. This acetylation promoted the production and release of neuronal SPMs, particularly 15-R-lipoxin A4 (15-R-LxA4), which is a potential resolver of inflammation. These SPMs not only effectively resolved neuroinflammation but also improved the microglia-mediated phagocytosis of Aβ plaques, which caused reduced Aβ burden in the brain. To the best of our knowledge, this is the first study to demonstrate a novel role for Sphk1 as an acetyltransferase for COX2 and describe the complex interactions between Sphk1 and COX2 activity. The study also postulates a bridging connection between Sphk1 and COX2 in maintaining CNS homeostasis and highlights the potential contribution of the Sphk1–COX2–SPM trilogy in AD (Figure 1a,c) [28].
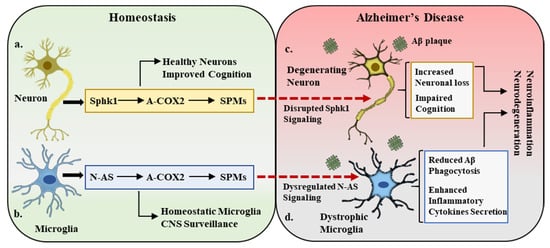
Figure 1. Sphk1–COX2–SPM trilogy in homeostasis and Alzheimer’s disease. (a). Under homeostatic conditions, neuronal cells express sphingosine kinase 1 (Sphk1), which is a potent regulator of COX2 acetylation (A-COX2) and subsequent SPM synthesis. This Sphk1–COX2–SPM trilogy maintains the central nervous system (CNS) homeostatic environment, improves cognition, and maintains healthy neurons. (b). In a parallel manner, the microglial compartment in the CNS expresses N-AS, which also acetylates COX2 and monitors SPM expression. This N-AS signaling maintains the microglial activity in balance and participates in CNS surveillance. (c,d). In Alzheimer’s disease pathology, amyloid beta (Aβ) deposition results in the disruption of Sphk1 signaling in neurons and N-AS expression in microglia, leading to neuronal loss (degenerating neurons), defective microglia (dystrophic microglia), cognitive impairment, and overall inflammatory and neurodegenerative environment.
Previous observations demonstrating neuronal Sphk1 acting as an acetyltransferase and regulator of COX2 acetylation and the subsequent synthesis of neuronal SPMs, which then act on microglia and modulate their phagocytic activity, indicate the indirect monitoring of microglial phagocytic activity by neurons. Although this neuronal Sphk1 and the microglial phagocytosis crosstalk are severely altered in AD pathology and subsequently exacerbate the disease, the direct controllers of the microglial phagocytosis of Aβ remain to be investigated. In a recent study by our research group, we reported the direct regulator of microglial phagocytic activity independent of neuronal tampering. The study reported that N-AS, an intermediate product in sphingosine metabolism, acetylates COX2 at serine 565 without neuronal involvement. It was observed that the Sphk1-regulated sphingosine metabolism involves the production of N-AS, wherein acetyl-CoA binds to the ATP-binding domain in Sphk1 and transfers this acetyl group to sphingosine, leading to the production of N-AS.
Under physiological conditions, both neurons and microglia synthesize N-AS, whose expression and function are affected by Aβ deposition in AD. Our study emphasized the importance of microglial-generated N-AS, as its levels were found to be severely reduced compared with those of neuronal N-AS in AD pathology. During AD progression, as Aβ plaques are increased, Sphk1 activity is disturbed, which results in decreased levels of N-AS, whereas microglial N-AS expression is reduced due to the lower availability of acetyl-CoA, an essential component in N-AS synthesis. Previous reports have demonstrated that AD mice models (2576 Tg and 3xTg) have reduced acetyl-CoA concentrations, which affects the microglial mitochondrial activity by inhibiting the pyruvate dehydrogenase complex, which ultimately results in defective microglia [29][30]. These findings are consistent with our microglial N-AS study, which urged us to investigate the role of N-AS in regulating microglial phagocytosis and its ultimate contribution to AD pathology.
It has also been demonstrated that a decrease in the synthesis of N-AS by acetyl-CoA-deficient microglia resulted in lower acetylation of COX2, which subsequently affected the release of SPMs and thus caused poor resolution of neuroinflammation and AD pathology. It has been observed that SPMs such as LXA4 and RvD1 play a significant role in maintaining the phagocytic functions of microglia [31][32]; therefore, N-AS-deficient microglia exhibit not only reduced SPM synthesis but also defective phagocytosis. Restoring the acetyl-CoA expression or exogenous administration of synthetic N-AS did rescue the AD pathology by improving COX2 acetylation, SPM secretion, and microglia-mediated Aβ phagocytosis. N-AS was able to restore the microglia’s multiple functional characteristics and also aid in reducing the gene expression of pro-inflammatory cytokines and limiting the infiltration of peripheral immune cells into the brain. This study reported a novel and direct regulator of COX2 anti-inflammatory actions and microglia functionality and demonstrated the need for further studies to explore the therapeutic potential of N-AS in AD (Figure 1b,d) [33].
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and affects ~1% of the population over 60 years of age [34]. Neuropathological hallmarks of PD include striatal dopamine deficiency due to neuronal loss in substantia nigra, and aggregation of α-synuclein, which results in the formation of lewy bodies. On the other hand, symptomatic hallmarks of PD include motor dysfunctions characterized by tremor, rigidity, slow moments, and impaired balance and gait. The pathophysiology of PD has been investigated from genetic predisposition, environmental factors, and aging aspects. The major mechanistic disruptions observed in PD pathology involve mitochondrial dysfunction, alterations in dopamine metabolism, generation of reactive oxygen species, microgliosis, and neuroinflammation, which altogether leads to neurodegeneration and the onset of motor dysfunctions [35].
In PD studies, sphingolipids have gained a great deal of attention in recent years, both at the genetic and metabolic levels, and will be discussed briefly in this section. Lipidomic analysis in postmortem PD brain tissue has shown an imbalance of ceramide and sphingomyelin levels along with increased levels of ceramides in blood plasma [36][37]. Glucocerebrosidase (GBA), a lysosomal enzyme that metabolizes glucosylceramide into free ceramide and glucose, is one of the top genetic contributors to PD development [38]. GBA mutations result in imbalanced ceramide levels and contribute to early PD development, rapid progression, and severe psychiatric symptoms. GBA is an important enzyme in α-synuclein degradation and is reported to protect against α-synuclein aggregation in the brain [39].
Some studies have reported a negative correlation between the aggregation of α-synuclein in neuronal cells and S1PR1 signaling, thus affecting the vesicular trafficking regulated by S1PR1 [40]. The studies by Joanna B Strosznajder’s group on the association between PD and sphingolipid metabolism have uncovered the potential role of Sphk1 in modulating the disease pathology. Their research reported that in a PD in vitro model of MTPT/MPP+ (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 1-methyl-4-phenylpyridinium), Sphk1 activity was significantly reduced, which enhanced α-synuclein secretion and the activation of genes associated with apoptosis. While the administration of PPX (pramipexole), a dopamine D2/D3 receptor agonist commonly used in PD therapy that acts via modulating Sphk1 activity, partially rescued the PD pathology [41][42]. These aforementioned studies altogether point towards the critical participation of different sphingolipid metabolites and enzymes in PD prognosis. Interestingly, sphingolipid studies in PD have shown similarities with AD as ceramide levels were increased, Sphk1 activity was significantly reduced, and Sphk1/S1P signaling was disrupted, as reported in AD as well. Based on the studies conducted on the Sphk1 and N-AS role in AD and the similarities between AD and PD in regard to sphingolipids, they may presents new targets (Sphk1 and N-AS) to explore in the PD pathogenesis.
Huntington’s disease (HD) is a progressive brain disorder caused by a dominant mutation in the Huntington (HTT) gene. HD is characterized by movement disorder such as random uncontrolled movements and imbalance; behavioral abnormalities like depression, anxiety, and psychosis; and cognitive impairment such as impaired learning and executive functions (problem solving, concentrating, and multitasking) and dementia [43]. Animal models of HD have reported that an imbalance in sphingolipid metabolite levels and enzyme activity occurs in the early stages of the disease’s development [44]. The study of HD postmortem brains and animal models has shown reduced Sphk1 activity in the cortex along with increased sphingosine-1-phsophate lyase 1 (SGPL1; an enzyme involved in the metabolism of S1P) [45]. The R6/2 mice model of HD has shown reduced levels of S1P in the brain, leading to abnormal neuronal activity, increased brain atrophy, motor dysfunction, and lower rates of survival [44]. All of these phenotypic and cellular pathologies were rescued upon FTY720 (S1PR agonist) administration [46].
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease, is a neurodegenerative neuromuscular disorder, characterized by the loss of motor neurons that control movement, muscle wasting, paralysis, and severe deregulation of lipid metabolism [47]. Mouse models of ALS display increased levels of ceramides in the spinal cord, immune cell dysregulation, along with disrupted exosome and lysosome secretion [48][49]. Disturbed ceramide, sphingosine, and sphingomyelin levels are reported to correlate with ALS severity together with Sphk1 activity and expression [49]. Altered levels of sphingomyelin and some other sphingolipids have been observed in the cerebrospinal fluid of ALS patients, pointing towards their clinical relevance. By contrast, hyperlipidemia (high levels of lipids in blood) appears to have a protective effect in ALS, and enhanced survival in an ALS mice model and slow progression of disease in ALS patients have been reported following hyperlipidemic diet consumption [50].
These studies of HD and ALS highlight the genetic and functional contribution of sphingolipid metabolites in the etiology of these disorders and why they should be explored in detail from a therapeutic perspective. Interestingly, HD and ALS have shown similar disturbances in sphingolipid metabolism to PD and AD. The investigation of Sphk1, N-AS, and COX2 acetylation in these disorders can unravel possible mechanisms and pave the way towards the design of relevant therapeutics for neurodegenerative disorders.