Zinc oxide nanoparticles have become one of the most popular metal oxide nanoparticles and recently emerged as a promising potential candidate in the fields of optical, electrical, food packaging, and biomedical applications due to their biocompatibility, low toxicity, and low cost. They have a role in cell apoptosis, as they trigger excessive reactive oxygen species (ROS) formation and release zinc ions (Zn2+) that induce cell death. The zinc oxide nanoparticles synthesized using the plant extracts appear to be simple, safer, sustainable, and more environmentally friendly compared to the physical and chemical routes. These biosynthesized nanoparticles possess strong biological activities and are in use for various biological applications in several industries. Initially, the present review discusses the synthesis and recent advances of zinc oxide nanoparticles from plant sources (such as leaves, stems, bark, roots, rhizomes, fruits, flowers, and seeds) and their biomedical applications (such as antimicrobial, antioxidant, antidiabetic, anticancer, anti-inflammatory, photocatalytic, wound healing, and drug delivery), followed by their mechanisms of action involved in detail.
1. Antibacterial Activity
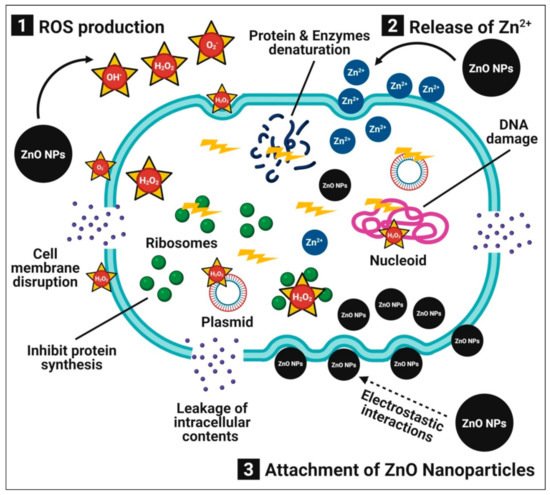
Bacterial infections are a major threat to humanity’s health. Researchers have focused on metal and metal oxide nanoparticles as antibacterial agents due to the increased antibiotic resistance in bacteria and the emergence of new strains [
2]. Zinc oxide nanoparticles have been studied extensively as antibacterial agents due to their unique physiochemical properties and increased surface areas [
4]. Furthermore, zinc oxide nanoparticles are both safe and compatible with the human body. Numerous publications describe the possible antibacterial mechanisms of zinc oxide nanoparticles, which involves (1) the production of ROS (i.e., OH
• (hydroxyl radical) and O
2−2 (peroxide)), which induces oxidative stress, cell membrane disruption, and DNA damage, resulting in the death of bacterial cells; (2) the dissolution of zinc oxide nanoparticles into the release of Zn
2+ ions, which interact with the bacterial cell, especially the cell membrane, cytoplasm, and nucleic acid, thus disintegrating the cellular integrity and resulting in bacterial cell death; and (3) direct interactions between zinc oxide nanoparticles and bacterial cell membranes through electrostatic forces that damage the plasma membrane and cause a leakage of intracellular components [
30] (
Figure 1).
Figure 1. Possible antibacterial mechanisms of plant-mediated zinc oxide nanoparticles.
In our previous study, zinc oxide nanoparticles synthesized from leaf extracts of
Ceropegia candelabrum with zinc nitrate (as a precursor) by the hydrothermal process resulted in nanoparticles of high purity, a hexagonal wurtzite shape, and 12–35-nm sizes [
5]. The biosynthesized zinc oxide nanoparticles significantly showed antibacterial potential against
Staphylococcus aureus,
Bacillus subtilis,
Escherichia coli, and
Salmonella typhi at 100 μg·mL
−1. One of our research groups also bio-fabricated zinc oxide nanoparticles from leaf extracts of
Cochlospermum religiosum to study their antibacterial potentiality [
82]. The bio-fabricated zinc oxide nanoparticles significantly exhibited the inhibition of Gram-positive (
B. subtilis and
S. aureus) and Gram-negative (
Pseudomonas aeruginosa and
E. coli) bacteria with minimum inhibitory concentrations (MIC) of 4.8–312.5 μg·mL
−1. The antibacterial potency of zinc oxide nanoparticles is probably due to their small size, which is more likely to damage the membrane, penetrate the cytoplasm, and produce numerous ROS that can damage the DNA and other cellular components of bacteria [
31,
80].
The difference in antibacterial activity can be associated with the structural and chemical compositions of the bacterial cell membranes [
2]. Sharma et al. [
127] studied the effect of the shape and size of zinc oxide nanoparticles synthesized by using
Aloe vera leaf extracts on their antibacterial activity and reported that cuboidal (40–45 nm)-shaped nanoparticles were shown to be more protuberant in antibacterial activity when compared to spherical (60–180 nm) and hexagonal (~65 nm)-shaped nanoparticles. Due to the small size of biosynthesized zinc oxide nanoparticles, they provide a high surface area, which leads to more interactions between nanoparticles and bacterial cells, which can be used as an antibacterial agent even at lower dosages. Biosynthesized zinc oxide nanoparticles initially interact with the bacterial plasma membrane, causing nanoparticles to enter the cytoplasm and release metal ions, thereby disrupting the membrane permeability and, finally, causing DNA damage, leading to the death of bacterial cells [
71]. The antibacterial activity demonstrated by zinc oxide nanoparticles synthesized from leaf extracts of
Cassia fistula and
Melia azadarach have a substantial ability to suppress clinical pathogens (such as
E. coli and
S. aureus) compared to traditional drugs, and it was concluded that the synthesis of nanoparticles employing the extracts of medicinal plants could be effective in the treatment of various human infectious diseases [
126]. Recently, Faisal et al. [
11] showed that 1% zinc oxide nanoparticles (1 mg mL
−1) synthesized from aqueous fruit extracts of
Myristica fragrans were found to display the maximum zone of inhibition against
Klebsiella pneumoniae,
E. coli,
P. aeruginosa, and
S. aureus.
2. Antifungal Activity
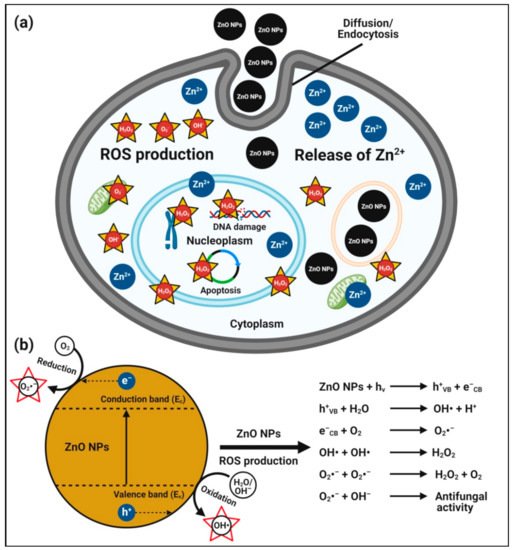
In addition to antibacterial activity, zinc oxide nanoparticles also possess antifungal activity against many harmful fungi and yeasts, making them promising antifungal food additives [
19,
103]. The possible mechanisms of antifungal activity of zinc oxide nanoparticles are described for zinc oxide nanoparticles that can enter fungal (conidial) cells by diffusion and endocytosis; they interfere in the mitochondrial function and promote ROS production Zn
2+ ion release inside the cytoplasm. The excess production of ROS and Zn
2+ ions released from zinc oxide nanoparticles can penetrate the nuclear membrane and cause irreversible DNA damage, inducing cell death [
134,
135] (
Figure 2). The role of the electronic band gap (E
g) property of zinc oxide nanoparticles with the redox potential (EH) of numerous ROS generation reactions have also been proposed [
134]. Zinc oxide nanoparticles electrons (e
−), when excited with energy higher than E
g, are promoted across the band gap energy to the conduction band edge (E
c), which creates a hole (h
+) in the valence band (E
v). The e
− in E
c and holes in E
v exhibit high reducing and oxidizing power, respectively. The e
− reacts with molecular oxygen (O
2) to produce superoxide anion (O
2•–) through sequential reduction reactions. The h
+ can extract e
− from water (H
2O) and/or hydroxyl ions to generate hydroxyl radical (OH
•).
Figure 2. Possible antifungal mechanism of plant-mediated zinc oxide nanoparticles. (a) Hypothetical anticandidal mechanisms of zinc oxide nanoparticles, and (b) plausible mechanisms of action of zinc oxide nanoparticle-induced ROS generation for their anticandidal activity.
The zinc oxide nanoparticles synthesized from flower extracts of
Nyctanthes arbor-tristis demonstrated antifungal activity against fungal phytopathogens (such as
Fusarium oxysporum,
Botrytis cinerea,
Penicillium expansum,
Alternaria alternata, and
Aspergillus niger) with the lowest MIC value of 16 μg·mL
−1, suggesting that they could be used to develop antifungal agents for commercial use in agriculture [
32]. Similarly, Khan et al. [
39] suggested that zinc oxide nanoparticles synthesized using
Trianthema portulacastrum extracts were found to exhibit antifungal properties against
Aspergillus niger,
A. flavus, and
A. fumigatus. Additionally, zinc oxide nanoparticles synthesized from different plant extracts such as
Beta vulgaris,
Cinnamomum tamala,
C. verum, and
Brassica oleracea were found effective against
Candida albicans and
A. niger [
25].
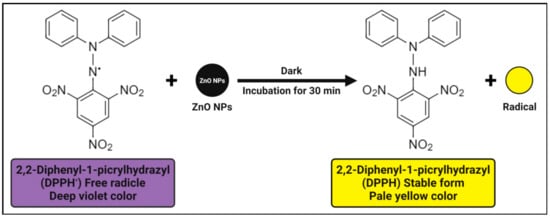
3. Antioxidant Activity
The antioxidant activity of zinc oxide nanoparticles is attributed to their smaller size, but another explanation may be a phenomenon in 2,2-diphenyl-1-picrylhydrazyl (DPPH) where the electron density is transferred from the oxygen atom to the odd electron located at the nitrogen atom, resulting in a decrease in the n → π* transition intensity at 517 nm. The antioxidant activity’s mechanism involves the unstable deep violet-colored methanolic solution of DPPH, which turns into stable pale-yellow color with the addition of zinc oxide nanoparticles due to their DPPH free radical scavenging activity through the transfer of an electron from the oxygen atom to the odd electron of a nitrogen atom, resulting in stable DPPH molecule formation (
Figure 3) [
83]. In other words, the antioxidant property of zinc oxide nanoparticles is determined by their ability to donate hydrogen. Furthermore, even in the absence of UV light, the formation of a large number of electron and hole pairs on the surfaces of zinc oxide nanoparticles results in a high redox potential that splits water (H
2O) molecules into hydroxyl (OH
•) and hydrogen (H
•) radicals, which are available for DPPH free radical reduction and DPPH molecule stability [
17,
83,
124].
Figure 3. Possible antioxidant mechanisms of plant-mediated zinc oxide nanoparticles.
Our previous study reported that the antioxidant activity of biosynthesized zinc oxide nanoparticles from
C. candelabrum showed significantly DPPH free radical scavenging activity from 0% to 55%, with an IC
50 value of 95.09 μg·mL
−1, compared with the 75% inhibition offered by ascorbic acid (a positive control) at 50 μg·mL
−1 [
5]. It is also reported that an increased antioxidant activity was observed with an increase in the zinc oxide nanoparticle concentration. According to the findings of Khan et al. [
39], the green synthesized zinc oxide nanoparticles had a strong antioxidant activity due to their charge density and capping materials on their surface. Alamdari et al. [
83] revealed that zinc oxide nanoparticles biosynthesized from leaf extracts of
Sambucus ebulus were found to exhibit H
2O
2free radical scavenging activity with an IC
50 value of 43 μg·mL
−1 and concluded that the presence of metal or Zn ions in the structure could enhance the antioxidant capabilities of the biosynthesized zinc oxide nanoparticles. Recently, Faisal et al. [
11] revealed that zinc oxide nanoparticles synthesized from the aqueous fruit extracts of
M. fragrans were found to show excellent free radical scavenging irrespective of the methods employed, viz., ABTS (82.12% TEAC), DPPH (66.3% FRSA), TAC (71.1 μg AAE·mg
−1), and TRP (63.41 μg AAE·mg
−1).
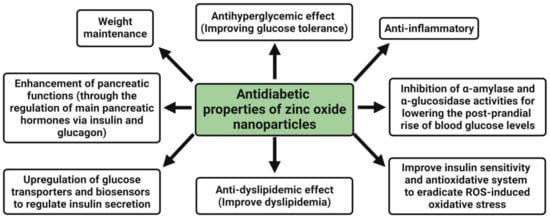
4. Antidiabetic Activity
Diabetes is a complex disease that requires an effective multifaceted treatment approach to care for patients. Antidiabetic medications are often used in conjunction with insulin or other drugs, resulting in higher medication costs overall. Zinc supplementation has been shown to improve glycemic regulation in diabetic humans and animals [
33,
66]. Furthermore, this metal can boost diabetic problems such as nephropathy and cardiomyopathy [
35]. Due to their capacity to deliver Zn
2+ ions, zinc oxide nanoparticles are currently being studied to treat diabetes and diabetic complications. The antidiabetic properties of zinc oxide nanoparticles are an antihyperglycemic effect, i.e., improving the glucose tolerance, enhancement of pancreatic functions (through the regulation of main pancreatic hormones, namely insulin and glucagon), upregulation of glucose transporters and biosensors to regulate insulin secretion, weight maintenance, anti-dyslipidemic effect (i.e., improve dyslipidemia), anti-inflammatory, inhibition of α-amylase and α-glucosidase activities for lowering the post-prandial rise of blood glucose levels, and improving the insulin sensitivity and antioxidative system to eradicate ROS-induced oxidative stress [
138] (
Figure 4).
Figure 4. Possible antidiabetic mechanism of plant-mediated zinc oxide nanoparticles.
The inhibition of carbohydrate metabolizing enzymes (such as α-amylase and α-glucosidase) is a unique strategy for controlling blood sugar, and it requires a large number of compounds to be evaluated in order to rule out inactive molecules and save time and money. In vitro α-amylase and α-glucosidase inhibitory studies revealed that zinc oxide nanoparticles synthesized from
Tamarindus indica extract had the highest inhibitory activity compared to the zinc oxide nanoparticles synthesized from other plant extracts [
22]. Additionally, Rajakumar et al. [
34] confirmed that the synthesized zinc oxide nanoparticles from
Andrographis paniculata leaf extract displayed better antidiabetic potential in terms of α-amylase inhibition activity with aIC
50 value of 121.42 μg·mL
−1. Similarly, the zinc oxide nanoparticles synthesized from
Costus igneus leaf extract were found to show more effective antidiabetic activity, wherein the percentage of inhibition ranged from 20% to 74% for α-amylase and 36% to 82% for α-glucosidase at aconcentration of 20–100 μg·mL
−1 of nanoparticles [
33]. Recently, Faisal et al. [
11] indicated that an excellent α-amylase (73.23%) and α-glucosidase (65.21%) inhibition activity at 400 μg·mL
−1 was offered by zinc oxide nanoparticles synthesized from aqueous fruit extracts of
M. fragrans and revealed that the bio-based nanoparticles could have strong antidiabetic properties and are considered a useful therapeutic agent for the treatment of diabetes as a replacement for expensive and ineffective medicines.
5. Anticancer Activity
The therapeutic regimes cannot distinguish between cancerous and normal cells, resulting in systemic toxicity and side effects. As a result, new chemotherapeutic agents with high selectivity for cancer cells are needed. Zinc deficiency initiates and facilitates the development of cancerous cells, as it is an important mineral that maintains homeostasis by controlling the enzyme activities in our body [
104]. Zinc is essential for the function of p-53 (a tumor suppressor gene) that controls apoptosis by activating the Caspase-6 (Casp6) enzyme. In addition, zinc oxide nanoparticles have a special electrostatic property that aids in the selective targeting of cancer cells. Anionic phospholipids are abundant on the surface of cancer cells, resulting in electrostatic attraction with zinc oxide nanoparticles, which encourages cancer cells to take up zinc oxide nanoparticles, resulting in cytotoxicity in cancer cells [
70,
129].
The small size of zinc oxide nanoparticles, on the other hand, aids in the permeation and retention of nanoparticles within tumorous cells, allowing them to act. The possible mechanisms behind zinc oxide nanoparticles’ selective pH-responsive cytotoxicity towards cancer cells are (1) the pH-dependent rapid dissolution of zinc oxide nanoparticles into the release of Zn
2+ ions under an acidic intracellular environment, which causes oxidative stress (via ROS production) and subsequent cell damage within cancer cells, and (2) the production of a large amount of ROS in cancer cells relative to normal cells; the elevated ROS level then causes mitochondrial dysfunction and activates the intrinsic mitochondrial apoptotic pathway (
Figure 5) (Sana et al. [
71]. ROS can also mediate cell death via extrinsic necrosis and apoptosis. Further, the overproduction of ROS leads to induced oxidative DNA damage and mitotic death and can also trigger autophagic/mitophagic cell death [
19,
26].
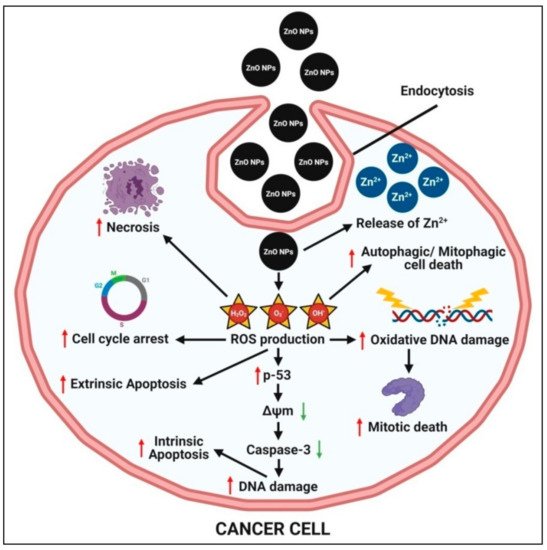
Figure 5. Possible anticancer mechanism of plant-mediated zinc oxide nanoparticles.
Zinc oxide nanoparticles have a particle size, shape, surface charge, concentration, and time-dependent cytotoxicity in cancer cells, and photo-irradiation with ultraviolet (UV) or near-infrared (NIR) lasers enhance its anticancer activity through a synergistic chemo-photodynamic effect [
29,
71]. The majority of the researchers also demonstrated that zinc oxide nanoparticles are less or nontoxic compared to normal cells in vitro when used in the same concentration range as cancer cells. Zinc oxide nanoparticles can cause systemic toxicity in tumor-bearing mouse models. However, using antioxidants in combination with zinc oxide nanoparticles may minimize the toxic side effects and increase the antitumor potential [
11]. Zinc oxide nanoparticles’ adverse toxic side effects can also be prevented or decreased by using cancer cell-specific ligands. While numerous reports on the anticancer properties of plant-mediated green synthesized zinc oxide nanoparticle in vitro systems are available, only a few studies in tumor-bearing mouse models have been performed [
93]. In vivo tumor models can be used to conduct further research using plant-mediated green synthesized zinc oxide nanoparticles with active cancer cell-targeting strategies.
The semiconducting nature of biosynthesized zinc oxide nanoparticles has been reported to induce cytotoxicity in cancer cells by forming ROS on the particle’s surface; the released Zn
2+ ions are dissolved in culture media, indicating the direct interaction of nanoparticles with a cancer cell membrane, resulting in oxidative stress and, ultimately, cancer cell death [
71]. Zinc oxide nanoparticles synthesized from the aqueous extract of
Deverra tortuosa exhibited enticing selective cytotoxic efficacy against two cancer lines (Caco-2 and A549), providing appealing ‘safer and cheaper’ alternatives to traditional therapy regimens [
99]. Faisal et al. [
11] used the
Streptomyces 85E strain to elucidate the protein kinase inhibition capability of zinc oxide nanoparticles synthesized from aqueous fruit extracts of
M. fragrans and concluded that the plant extracts provide crucial capping and stabilizing agents to biogenic nanoparticles, which are responsible for their anticancer properties. Recently, Umamaheswari et al. [
29] reported that the anticancer activity of zinc oxide nanoparticles biosynthesized from leaf extracts of
Raphanus sativus was higher after treating the A549 cell lines, indicating that cancer drugs can be prepared using this environmentally friendly method. The DNA damage pathways, paraptosis, autophagy, radio sensitizing, aberrant cellular metabolism, overcoming chemo resistance, oxidative stress modulation, induction of apoptosis, arresting of the cancer cell cycle, anti-invasion, and metastasis are some of the possible anticancer mechanisms that have been reported so far [
139].
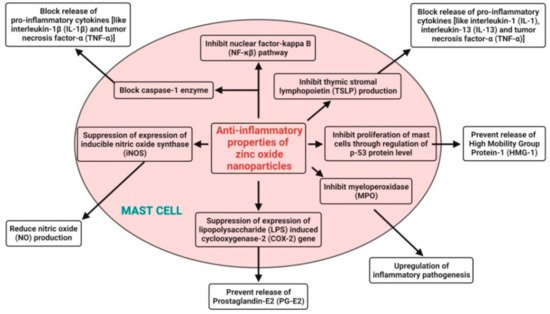
6. Anti-Inflammatory Activity
Nanoparticles have been widely exploited as a potential anti-inflammatory agent in recent years. The large surface area-to-volume ratio causes nanoparticles to have more surface reactive properties, resulting in more interactions with the cell membrane and easier transport within the membrane [
34,
97,
125]. Zn is easily transported through the cell membrane because of the nanosizes of zinc oxide nanoparticles. The anti-inflammatory mechanisms adopted by zinc oxide nanoparticles involve the release of Zn
2+ ions from the dissolution of zinc oxide nanoparticles and subsequent block of the release of proinflammatory cytokines (like interleukin-1 (IL-1), interleukin-1β (IL-1β), interleukin-13 (IL-13), and tumor necrosis factor-α (TNF-α)) in mast cells; they suppress the expression of the lipopolysaccharide (LPS)-induced cyclooxygenase-2 (COX-2) gene in macrophages to prevent the release of prostaglandin-E2 (PG-E2), suppress the expression of inducible nitric oxide synthase (iNOS) to reduce nitric oxide (NO) production, inhibit myeloperoxidase (MPO) for the upregulation of inflammatory pathogenesis, inhibit the nuclear factor-kappa B (NF-κβ) pathway and block the caspase-1 enzyme in activated mast cells, inhibit the proliferation of mast cells through the regulation of the p-53 protein level to prevent the release of High Mobility Group Protein-1 (HMG-1), and also, inhibit thymic stromal lymphopoietin (TSLP) production by primary epithelial cells [
140] (
Figure 6).
Figure 6. Possible anti-inflammatory mechanisms of plant-mediated zinc oxide nanoparticles.
The zinc oxide nanoparticles synthesized from
Polygala tenuifolia root extract displayed excellent anti-inflammatory activity, even up to 1 mg·mL
−1, by suppressing the LPS-induced mRNA and protein expressions of iNOS; COX-2; and anti-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in LPS-stimulated RAW 264.7 murine macrophage cells in a dose-dependent manner [
17]. Agarwal and Shanmugam [
21] also revealed that green synthesized zinc oxide nanoparticles from
Kalanchoe pinnata leaf extract were found to be biocompatible up to 1 mg·mL
−1 and to have an anti-inflammatory effect by inhibiting the production and release of proinflammatory mediators such as IL-1β, IL-6, TNF-α, and COX-2. Likewise, Liu et al. [
125] proved the anti-inflammatory effects of synthesized zinc oxide nanoparticles from
Vernonia amygdalina leaf extract against different pain and inflammation-induced mice. Moreover, the synthesized zinc oxide nanoparticles were found to exhibit potent anti-inflammatory effects via reducing the inflammatory response and proinflammatory cytokines level in mice.
7. Photocatalytic Activity
To prevent water pollution, various semiconductors, including zinc oxide, have been evaluated as photocatalysts and have proposed a viable solution. Zinc oxide nanoparticles synthesized from biological origin have shown a remarkable photocatalytic degradation of several dyes, including methylene blue, methyl orange, etc., due to their intrinsic ability to absorb UV irradiation and optical transparency. Since methylene blue is a major water pollutant emitted by industries such as textiles and is widely recognized as a traditional organic pollutant, these zinc oxide nanoparticles have gained much publicity due to environmental concerns [
92,
110,
130]. Furthermore, the toxic effluents discharged by the textile industry have harmed marine life and the ecosystem, resulting in a serious environmental crisis. The bio-fabricated zinc oxide nanoparticles were utilized as a photocatalyst to degrade carcinogen organic dyes where the structure, particle size, crystallinity, photocatalyst band gap, surface area, and other factors were considered [
40,
96].
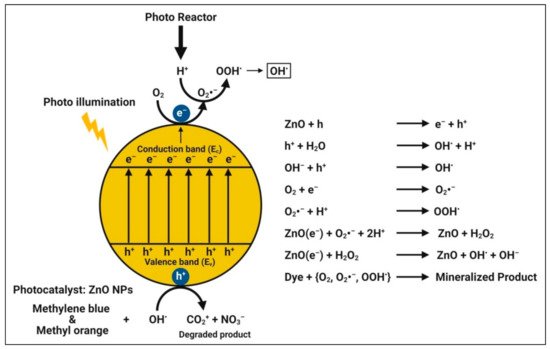
The mechanism of photodegradation is that, when semiconductors (catalysts) consume energy (photons) greater than their bandgap energy, charge separation occurs due to an electron jumping from the valence band to the conduction band of the catalyst, resulting in the creation of a hole in the valence band [
64,
70,
108,
122]. The created electron-hole pairs diffuse onto the photocatalyst’s surface and participate in the chemical reaction with the electron donor and acceptor when illuminated by light stronger than the band gap energy. With super strong oxidization, certain free electrons and holes convert the surrounding oxygen or water molecules into highly reactive unstable OH
• free radicals. When zinc oxide nanoparticles absorb photons with energies greater than their band gap energy from illumination, photocatalytic reactions begin to degrade the dyes [
83] (
Figure 7). Therefore, among the other zinc species in aqueous dye solutions under the experimental conditions, Zn
2+ ions must be the most abundant, followed by Zn(OH)
+, Zn(OH)
3−, and Zn(OH)
42–.
Figure 7. Possible photocatalytic mechanism of plant-mediated zinc oxide nanoparticles.
In the presence of solar light as a catalyst, the biosynthesized zinc oxide nanoparticles showed a strong degradation capability for Synozol navy blue K-BF, and the hypothesized degradation mechanism yielded the formation of hydroxyl radical and dye degradation into smaller fragments [
39]. Alamdari et al. [
124] indicated that zinc oxide nanoparticles biosynthesized from the leaf extract of
S. ebulus showed the maximum degradation of methylene blue dye noticeably around 80% after 200 min of UV radiation. Recently, Faisal et al. [
11] reported that zinc oxide nanoparticles biosynthesized from aqueous fruit extracts of
M. fragrans were used as photocatalytic agents, resulting in 88% of methylene blue dye degradation in 140 min of UV light illumination. In addition, Vinayagam et al. [
130] reported that zinc oxide nanoparticles synthesized from
Bridelia retusa leaf extract promoted the photocatalytic degradation of Rhodamine B dye by up to 94.74% within 165 min of solar irradiation. The crystalline and mesoporous natures, high surface area, and good electron acquiring features of the zinc oxide nanoparticles might be attributed to the accelerated degradation. Furthermore, the improved photocatalytic activity could be attributed to the stabilizing phytocompounds.
8. Wound-Healing Activity
Since our skin is our body’s largest organ, it protects us from external attack, and any damage to it results in a wound. The healing of a wound takes time and can be slowed by microbial infection (
P. aeruginosa and
S. aureus). Nanoparticles have antibacterial properties and produce hydrogen peroxide, which causes cell damage [
87]. Metal oxide nanoparticles can be utilized to destroy the pathogenic species and thereby improve wound healing [
67]. Zinc oxide nanoparticles synthesized biologically from plant sources can be tremendously used as a wound-healing agent for treating general wounds and wounds caused due to burning [
67,
87,
114]. Khatami et al. [
114] indicated that zinc oxide nanoparticles (about 26 nm in size) synthesized from
Prosophis fracta and coffee were found to impregnate on cotton wound bandages, conferring patches with a strong antimicrobial effect. Hence, they can potentially be used for treating and covering infection-sensitive wounds, namely diabetic or burns wounds. Shao et al. [
87] biosynthesized zinc oxide nanoparticles from a
Barleria gibsoni leaf extract, which acted as efficient and superior tropical antimicrobial formulations for the healing of burn infections by exhibiting a remarkable wound-healing potential in rats. Kiran Kumar et al. [
142] studied zinc oxide nanoparticles synthesized from a
Raphanus sativus root extract and showed their antimicrobial property against
Escherichia fergusonii and
E. coli strains that can be further explored for wound-healing potential. Apart from these, many reports have also indicated that the application of zinc oxide nanoparticles/or their biocomposites, irrespective of their application, possessed potential wound-healing ability and help in wound healing [
143,
144,
145,
146].
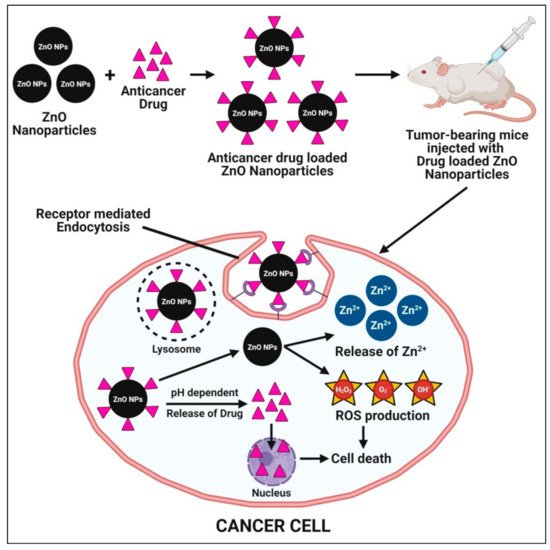
9. Targetted Drug Delivery System
Zinc oxide nanoparticles have been considered as a potential candidate for targeted drug delivery because of their ease of synthesis from low-cost metal precursors, biocompatibility, and successful cellular internalization via endocytosis. Nanoparticles’ smaller particle sizes and larger surface areas make it easier for them to penetrate cell membranes and be absorbed into cells, resulting in better distribution. As a result, nanoparticles are commonly used in drug delivery systems, where they are loaded with drugs that reach their targets in appropriate quantities and then guide the drug release from the nanoparticles [
51]. The effect of zinc oxide nanoparticles on drug delivery revealed that these nanoparticles aid in the rapid release of the drug at first and then transition to regulate the release over the treatment period [
95]. The mechanisms underlying the enhanced cytotoxic potential of anticancer drug-loaded zinc oxide nanoparticles involve the pH-dependent release of targeted drug and zinc oxide nanoparticles in the cytoplasm once the drug-loaded zinc oxide nanoparticles enter through receptor-mediated endocytosis. The release of targeted anticancer drugs act upon cancer cells and cause cell death. Additionally, zinc oxide nanoparticles release Zn
2+ ions and produce excessive ROS (oxidative stress) and, subsequently, lead to cancer cell death (
Figure 8).
Figure 8. Possible mechanisms of plant-mediated zinc oxide nanoparticles in a targeted drug delivery system.
Vimala et al. [
52] used green synthesized doxorubicin (DOX)-loaded zinc oxide nanoparticles with
Borassus flabellifer fruit extract in a drug delivery system for anticancer therapy. The synthesized DOX–zinc oxide nanoparticles showed dose-dependent cytotoxicity against the human breast cancer MCF-7 and colon cancer HT-29 cell lines, with an inhibitory concentration (IC
50) of 0.125 μg·mL
−1. Additionally, the toxicity assessment in vivo showed that these DOX–zinc oxide nanoparticles had low systemic toxicity in the murine model system. The low toxicity and high anticancer therapy efficacy of DOX–zinc oxide nanoparticles were found to provide strong evidence for plant-mediated green synthesized zinc oxide nanoparticles as promising candidates in drug delivery systems. Akbarian et al. [
95] successfully loaded an important anticancer drug (Paclitaxel, PTX) on chitosan-coated zinc oxide nanoparticles, which were synthesized from the ethanolic leaf extract of
Camellia sinensis and evaluated their biological activity as a drug delivery system on breast cancer cell lines (MCF-7). The PTX loaded on the chitosan-coated zinc oxide nanoparticles were found to show acytotoxic (anticancer) effect on cancerous MCF-7 cell lines and reduced the cytotoxic effect on normal fibroblast cell lines. Despite the reports on the use of zinc oxide nanoparticles in targeted drug delivery systems, further research is required to develop zinc oxide nanoparticles synthesized from plant sources as a flexible drug delivery system for targeted delivery. Passive targeting administered nanoparticles preferentially accumulate in solid tumors with discontinuous endothelium based on the enhanced permeability and retention (EPR) phenomenon, which is directly dependent on the size of the particles, which is smaller than the tumor inter-endothelial gap cut-off size. Apart from this, the two fundamental characteristics of neoplastic tissues, such as leaky vasculature and impaired lymphatic drainage, may also facilitate nanoparticle targeting [
147].
10. Tissue Engineering and Regenerative Medicine
Zinc oxide nanoparticles have emerged as promising materials for tissue engineering and regenerative medicine applications, which can help to promote tissue regeneration while reducing immune responses and preventing infections because of their antineoplastic, angiogenic, ultraviolet scattering, and wound-healing properties [
148]. The osteogenesis and angiogenesis effects of zinc oxide nanoparticles have been effectively proven in several investigations due to their extraordinary antibacterial activity, and inexpensive cost [
149,
150]. Zinc oxide nanoparticles are also found to promote cell growth, proliferation, differentiation, and metabolic activity in a variety of cell lines for tissue engineering and regenerative medicine applications [
151]. As a result, the use of plant-mediated zinc oxide nanoparticles in tissue engineering and regenerative medicine has become even more specialized and utilizing the innovative concept that zinc oxide nanoparticles’ proangiogenic properties might be tremendously advantageous in increasing the integration of advanced scaffolds into host tissue, as evidenced by in vitro and in vivo experiments.
Shubha et al. [
152] synthesized zinc oxide nanoparticles using Gallic acid isolated from the aqueous extract of
Phyllanthus emblica and reported that the smaller-sized plant-mediated zinc oxide nanoparticles were found to cause noticeably lesser toxicity than clinically advocated zinc oxide nanoparticles. Since they employed 3T3 fibroblasts from Balb mice, which are important connective tissue cells that aid in tissue repair and regeneration, and because these zinc oxide nanoparticles are benign to the cells, they can be used close to connective tissue cells. Shafique et al. [
153] reported that biosynthesized zinc oxide nanoparticles from a
Cymbopogon citratus leaf extract were found, showing a promising callogenesis and regeneration frequency at their optimum concentrations (20 mg·L
−1 and 30 mg·L
−1 in the internodes and seeds, respectively) in
Panicum virgatum, and it was confidently believed that this finding would open new doors in tissue culture technology by increasing the frequency of plant in vitro regeneration in different plant groupings.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13101662

 Encyclopedia
Encyclopedia


