Ayahuasca is a hallucinogenic botanical beverage originally used by indigenous Amazonian tribes in religious ceremonies and therapeutic practices. While ethnobotanical surveys still indicate its spiritual and medicinal uses, consumption of ayahuasca has been progressively related with a recreational purpose, particularly in Western societies. The ayahuasca aqueous concoction is typically prepared from the leaves of the N,N-dimethyltryptamine (DMT)-containing Psychotria viridis, and the stem and bark of Banisteriopsis caapi, the plant source of harmala alkaloids. DMT is a classical hallucinogen and a simple tryptamine, that acts as an agonist of the serotonin (5-HT) receptor 5-HT2A, through which it exerts its effects in the central nervous system (CNS).
- Hallucinogens
- Ayahuasca
- Psychotria viridis
- Serotonin receptors
1. Introduction
Naturally-occurring hallucinogens have been used for millennia by many indigenous cultures, not only in religious and spiritual ceremonies but also due to their medicinal benefits [1,2]. Their use in modern cultures witnessed a rapid spread in the 1960s and 1970s, mainly due to their popularity as recreational drugs [3]. These compounds are characterised by an overall capacity to induce mood alterations, variations in a person’s perception, mainly visual, and altered thought with overwhelming intellectual or spiritual insight, similar to what is experienced only through dreams or at times of religious exaltation [4,5]. In addition to the term hallucinogen (meaning that the drug produces hallucinations, while devoid of psychedelic effects at typical low doses) [1], other designations such as entheogen, mysticomimetic, or psychotogen were also used to label these compounds [2]. Ultimately, the most commonly used designation to describe the nature of these drugs is psychedelic (“mind revealing” or “mind-manifesting”), a term that do not focus on one specific effect [1]. Long-time classified as dangerous drugs by the media and regulatory agencies worldwide, psychedelics are very frequently devoid of serious toxic effects [3], psychoactivity being produced at low doses, usually insufficient to induce toxicity in a mammalian organism [1,6]. Additionally, their use does not lead to dependence, nor they are consumed for long periods of time [7,8], with unusual reports of chronic use [9].
Ayahuasca, a Quechua word that means “vine of the souls” [4,12], and also known as yagé, hoasca, caapi, daime, and natem [13], is a hallucinogenic herbal preparation with a long traditional use both for therapeutic and divination purposes by indigenous tribes of the Amazon Basin, who consider it a sacred beverage [14]. The pharmacological activity is mostly attributed to the allegedly synergistic interaction between the psychoactive alkaloids of Psychotria viridis and of the Banisteriopsis caapi vine [12], the most commonly used admixture plants. P. viridis leaves contain high amounts of DMT, a potent short-acting psychedelic alkaloid that is inactivated per os due to first-pass metabolism by monoamine oxidase A (MAO-A) enzymes. β-Carboline alkaloids, such as harmine and harmaline, occur in the stem and bark of B. caapi and are potent and reversible inhibitors of MAO-A (MAO-AI), also having psychotropic properties [6,15]. Concomitant intake through ayahuasca allows the delivery of high levels of DMT to the CNS, enabling a potent psychotropic action [16]. As first reported by the Hungarian chemist and psychiatrist Stephen Szara, who self-administered 1 mg/kg extract through intramuscular injection [17], DMT psychotropic effects include euphoria, visual hallucinations, spatial distortions, and speech disturbance, very similar to what had been previously described for LSD [18].
The two syncretic Brazilian churches União do Vegetal (UDV) and Santo Daime have an historic use of ayahuasca as a sacrament in religious ceremonies [12]. Such religious groups use ayahuasca both as a healing tool and as a way to “get in touch with the divine realm” [19]. The growing number of religious institutions as well as centres of alternative therapies that are allowed to use ayahuasca, led to a worldwide spread consumption by users seeking a spiritual experience or a direct psychedelic effect [20,21,22]. As such, its recreational use is sharply rising, with a global scale online survey showing an increased popularity on DMT consumption [23]. Such growing interest urges the understanding of the overall pharmacological action and safety of ayahuasca and its single bioactives. Furthermore, ayahuasca has also been brought into the spotlight by researchers due to the potential therapeutic benefits deriving from the modulation of the serotonergic system.
2. Pharmacodynamics
It has long been believed that the effects of serotonin were mimicked by psychedelic drugs [2]. In fact, classic hallucinogens like DMT act as agonists of serotonin receptors, leading to increased brain levels of serotonin [96,97,98]. Preliminary findings [99] suggested that hallucinogenic drugs exert their effects by acting on a specific group of serotonin receptors, the 5-HT2 receptors. Subsequent studies further corroborated the involvement of the serotonin 5-HT2A receptor in the visual hallucinations caused by classic psychedelics [6,100], which are nowadays considered a key site for their action [1].
DMT has affinity for a variety of neuroreceptors, including the types 1A, 1B, 1D, 2A, 2B, 2C, 6 and 7 of 5-HT receptor, with reports of at least a partial agonism of the 5-HT1A (Ki = 183 nM), 5-HT2A (Ki = 127 nM), and 5-HT2C (Ki = 360 nM) [56,101,102]. In a study with rat fibroblasts expressing the 5-HT2A or the 5-HT2C receptors, DMT was characterised as a full agonist of the former, but only a partial agonist of 5-HT2C, profound desensitisation over time only being observed with the 5-HT2C receptor [102]. Contrary to other psychedelics, for which repeated administration leads to a very rapid development of tolerance [6], DMT does not appear to induce human tachyphylaxis [103]. In this sense, desensitisation of 5-HT2C in response to repeated DMT administration in humans suggests that its mechanism of action is mainly mediated by the interaction with 5-HT2A receptors. Additionally, the higher distribution of 5-HT2A receptors in the cortex (the brain area where hallucinogens are thought to act) in comparison with 5-HT2C reaffirms the higher relevance of 5-HT2A receptors as mediators of psychedelic effects [104]. Of note, it has been suggested that the high affinity of DMT for 5-HT2A receptors might derive from the small N-methylated moieties [105].
As most 5-HT receptors, 5-HT2A is a member of the superfamily of G protein-coupled receptors [1], whose ligand-dependent stimulation leads to the activation of a series of intracellular signalling pathways (Figure 1) [6]. The receptor is found widespread in the mammalian brain throughout the cortex, striatum, hippocampus, and amygdala [106].
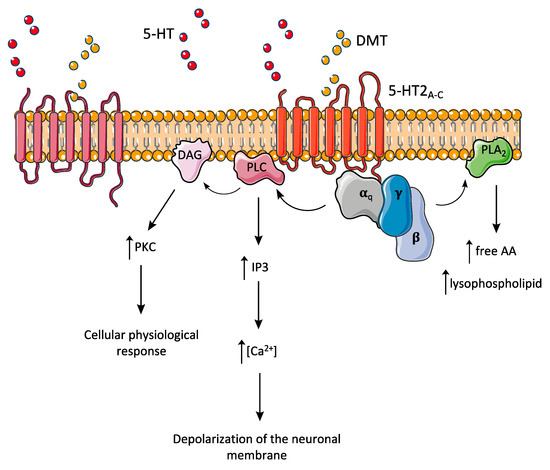
As reported in several studies, activation of 5-HT2A by psychedelics can potentiate the expression of genes encoding transcription factors, such as c-fos, egr-1 and egr-2 [107,108], known to be associated with synaptic plasticity, memory, and attention [109,110,111]. Although the acute effects of DMT/ayahuasca or other psychedelics are related to the immediate electrophysiological changes after 5-HT2A receptor activation, these longer-lasting implications in transcription modulation may trigger the differences observed in long-term users of ayahuasca [35,112]. Furthermore, transcription modulation may underlie the known antidepressant effects of 5-HTergic psychedelics [113], including those suggested to derive from ayahuasca consumption [114,115].
The 5-HT2C receptor is also coupled to Gq, with increased hydrolysis of phosphatidylinositol membrane lipids after activation [102]. However, this agonist effect is not considered to play a main role in the production of DMT effects [102,116].
DMT is also an agonist of the pre-synaptic 5-HT1A receptor [6]. This receptor is coupled to Gi proteins, being abundant in the somas and dendrites of serotonergic neurons in the raphe nuclei of the brainstem [35,117]. Contrary to 5-HT2A/2C, this receptor is involved in inhibitory neurotransmission, leading to reduced 5-HT release in other brain regions following its activation [56,118]. Desensitisation of these receptors can be achieved with chronic consumption of antidepressants, resulting in the restoration of the normal activity of 5-HT in neurons [119]. Due to this, the reported anxiolytic and antidepressant properties of ayahuasca and/or DMT [114] are thought to be also related with agonism towards 5-HT1A receptors [56].
Little is known of the consequences of the interaction between DMT and the 5-HT1D/6/7 receptors. However, it would be important to evaluate the potential role of this interaction in the promotion of some behavioural and therapeutic effects of ayahuasca, since various aspects of learning, memory, neuroplasticity, and cognition, have been associated with 5-HT6/7 receptors [120,121,122,123].
Additionally, DMT is also known to act as a substrate of the vesicular monoamine transporter 2 (VMAT2) [124] and 5-HT transporter (SERT) [124,125]. As such, DMT effects may involve a complex and intricate interaction of multiple systems and cannot be solely explained by its action towards the 5-HT receptors. In fact, DMT also binds and activates non-serotonergic receptors [55]. DMT is one of the only known endogenous agonists of the sigma-1 receptor (σ1R) [126]. These receptors are expressed on the mitochondria-associated endoplasmic reticulum membrane of the brain, but also in lung, prostate, colon, ovary, breast, and liver cells [22]. After stimulation, sigma-1 receptor modulates calcium signalling through interaction with inositol-1,4,5-triphosphate (IP3) receptors at the endoplasmic reticulum membrane [127]. Additionally, it can migrate to the cell’s plasma membrane, where it interacts with/inhibit several ion channels, including voltage-gated sodium and potassium channels [22,35,128]. The relation between the activation of this receptor and the psychedelic effects of DMT is, however, still debatable [6], as other substances that are devoid of hallucinogenic properties (e.g., cocaine) have also been known to exhibit a similar binding ability [129]. Since sigma-1 receptors have been targeted for the treatment of some neurological disorders [130,131], therapeutic properties of ayahuasca and/or DMT, including the treatment of depression, anxiety, schizophrenia, and promotion of neural plasticity, could be reasonably hypothesized to be, at least in part, associated with this receptor [56,132]. Their involvement in the antidepressant effects attributed to ayahuasca is supported by the mechanism of action of certain antidepressants (e.g., fluvoxamine) that also stimulate sigma-1 receptors [35,133].
DMT also acts as an agonist at the trace amine-associated receptor type 1 (TAAR1), whose activation leads to an increase in cAMP production [134]. This activation was proposed to result in a suppression of psychosis and induction of a relaxed mental state [135]. The affinity of DMT to TAAR1 can be related to its anxiolytic properties [136], suggesting a potential therapeutic utility for DMT and ayahuasca.
Although DMT is considered to be the main alkaloid responsible for ayahuasca psychotropic properties, β-carbolines are also psychoactive, and, if in sufficient amount, may directly contribute to behavioural effects in consumers [137,138]. They can induce psychological and physiological effects via modulation of the levels of amine neurotransmitters in the CNS, either by inhibiting their metabolism or by direct interaction with specific receptors [22,139]. The β-carbolines harmine and harmaline act as selective and reversible MAO-AI, while THH acts as an inhibitor of the 5-HT reuptake (Figure 2) [140], with weaker or absent action upon MAO-A [13,29]. Hallucinogenic effects are also reported following the administration of β-carbolines without DMT, which could also be a result of their direct binding to 5-HT2A or 5-HT2C receptors [138,141].

Another main mechanism of action that has been proposed includes the stimulation of dopamine (DA) efflux (Figure 2) [142]. Accordingly, harmine and harmaline seem to cause increased DA release, affecting the pathways mediated by this neurotransmitter [22]. Less-studied mechanisms have also been proposed [22]. It was suggested that harmine inhibits the DA transporter (DAT) [143], resulting in high levels of DA in the synaptic cleft, thus modulating dopaminergic neurotransmission [144]. Additionally, harmine is a potent inhibitor of tyrosine-phosphorylation-regulated kinase 1A (DYRK1A), an enzyme responsible for modulation of DAT membrane trafficking (Figure 2), further corroborating the possible role of harmine in the rate of DA reuptake [142].
This entry is adapted from the peer-reviewed paper 10.3390/ph13110334
